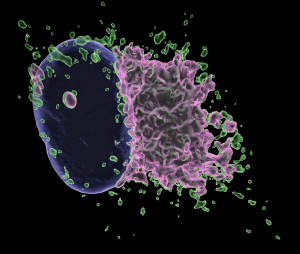
Image courtesy Hoxhaj Lab: NADK2 regulates mRNA translation in mitochondria (green: mitochondria, pink: mitochondrial translation)
Mitochondrial NADPH — a metabolite and essential electron donor — drives mitochondrial fatty acid synthesis (mtFAS) and protein lipoylation, two processes critical to cellular energy metabolism, according to new research from Children’s Medical Center Research Institute at UT Southwestern (CRI) published in Nature Cell Biology.
For decades, the mtFAS pathway has been poorly understood in mammals due to a lack of tools to measure this process. Gerta Hoxhaj, Ph.D., Assistant Professor in CRI, and first author Dohun Kim, B.S., a graduate student researcher in the Hoxhaj Lab, in collaboration with researchers at the U.S. Department of Agriculture (USDA), have developed a new biochemical method to directly measure mtFAS activity in mammalian cells.
“We hope this new method will open new avenues for exploring this fascinating pathway,” Mr. Kim said. “Our study sheds light on a long-overlooked, yet essential, metabolic pathway.”
Mitochondria have their own specialized fatty acid synthesis pathway, distinct from the well-characterized cytosolic process. While cytosolic fatty acid synthesis supplies lipids for membrane formation and energy storage, the mtFAS pathway serves a different purpose: it fuels mitochondrial energy production by enabling a rare lipid modification known as protein lipoylation, which is critical for several key mitochondrial enzymes.
Photo courtesy the Vilcek Foundation: Gerta Hoxhaj, Ph.D., Assistant Professor in CRI, and first author Dohun Kim, B.S., a graduate student researcher in the Hoxhaj Lab.
Using their newly-developed tool, CRI researchers discovered mitochondrial NADPH, produced by the enzyme NADK2, powers the synthesis of fatty acyl chains which are covalently attached to a protein called ACP. This process enables efficient mitochondrial translation, protein lipoylation, and oxidative metabolism.
“Our findings establish mitochondrial NADPH as a central metabolic currency for sustaining oxidative mitochondrial metabolism,” Dr. Hoxhaj said. “Many tumors — particularly in lung cancer — amplify NADK2, underscoring the importance of mitochondrial NADPH and its role in supporting mtFAS in cancer cell metabolism. This discovery opens new directions for understanding how mtFAS is regulated and contributes to disease.”
This study builds on prior Hoxhaj Lab research which showed mitochondrial NADPH is essential for proline synthesis. Now, CRI scientists have expanded the repertoire of mitochondrial NADPH-dependent processes to include mitochondrial fatty acid synthesis and protein lipoylation to maintain cellular health.
Dr. Hoxhaj is a member of the CRI Genetic and Metabolic Disease Program. She is a Pew-Stewart Scholar, Cancer Prevention and Research Institute of Texas (CPRIT) Scholar in Cancer Research, V Foundation Scholar, American Cancer Society Scholar, 2025 Texas Academy of Medicine, Engineering, Science and Technology (TAMEST) Mary Beth Maddox Award and Lectureship for her research in cancer metabolism, and a 2024 recipient of the Vilcek Prize for Creative Promise in Biomedical Science.
In addition to CRI, Dr. Hoxhaj is an Assistant Professor of Biochemistry and Pediatrics at UT Southwestern and member of the Cellular Networks in Cancer Research Program at the Harold C. Simmons Comprehensive Cancer Center.
Mr. Kim is a recipient of a CPRIT training grant.
This research was supported by grants from the National Institutes of Health, Welch Foundation, CPRIT, and USDA National Institute of Food and Agriculture Award.