CRI researchers, international colleagues identify PEX39, which facilitates peroxisomal protein import
PEX39 is the first new human peroxisomal biogenesis protein, a peroxin or PEX, to be identified in the past 20 years, according to new research by Children’s Medical Center Research Institute at UT Southwestern and international colleagues published in Nature Cell Biology.
This discovery explains how cells assemble peroxisomes, essential organelles that allow cells to metabolize certain fats and other metabolites. The work was performed by Ralph J. DeBerardinis, M.D., Ph.D., Professor and Director of the Eugene McDermott Center for Human Growth and Development, as well as Professor in CRI and Director of the CRI Genetic and Metabolic Disease Program, and first author Walter W. Chen, M.D., Ph.D., a postdoctoral fellow in the DeBerardinis Lab and Instructor and Attending Physician of Neonatal-Perinatal Medicine in Pediatrics at UT Southwestern Medical Center.

Ralph J. DeBerardinis, M.D., Ph.D., (left) Professor and Director of the Eugene McDermott Center for Human Growth and Development, as well as Professor in CRI and Director of the CRI Genetic and Metabolic Disease Program, with study first author Walter W. Chen, M.D., Ph.D., postdoctoral fellow in the DeBerardinis Lab and Instructor and Attending Physician of Neonatal-Perinatal Medicine in Pediatrics at UT Southwestern Medical Center.
To thoroughly analyze the function of PEX39, Drs. DeBerardinis and Chen collaborated with Daniel Wendscheck, M.Sc., a graduate student in the lab of Bettina Warscheid, Ph.D., Professor and Chair of Biochemistry at the University of Würzburg in Germany, and Tony Rodrigues, Ph.D., a postdoctoral fellow in the lab of Jorge Azevedo, Ph.D., Professor of Molecular Biology at the University of Porto in Portugal.
Together these scientists utilized biochemistry and functional assays in yeast and human cells to show the uncharacterized human protein C6ORF226, called Yjr012c in yeast, is actually a peroxin, which they renamed PEX39.
Peroxisome assembly requires peroxins, many of which are necessary for importing metabolic enzymes into peroxisomes.
Peroxisomal protein import is an active area of research because peroxisomes have the unusual ability to import completely folded proteins, as compared to other well-researched organelles such as mitochondria, which can only import unfolded proteins.
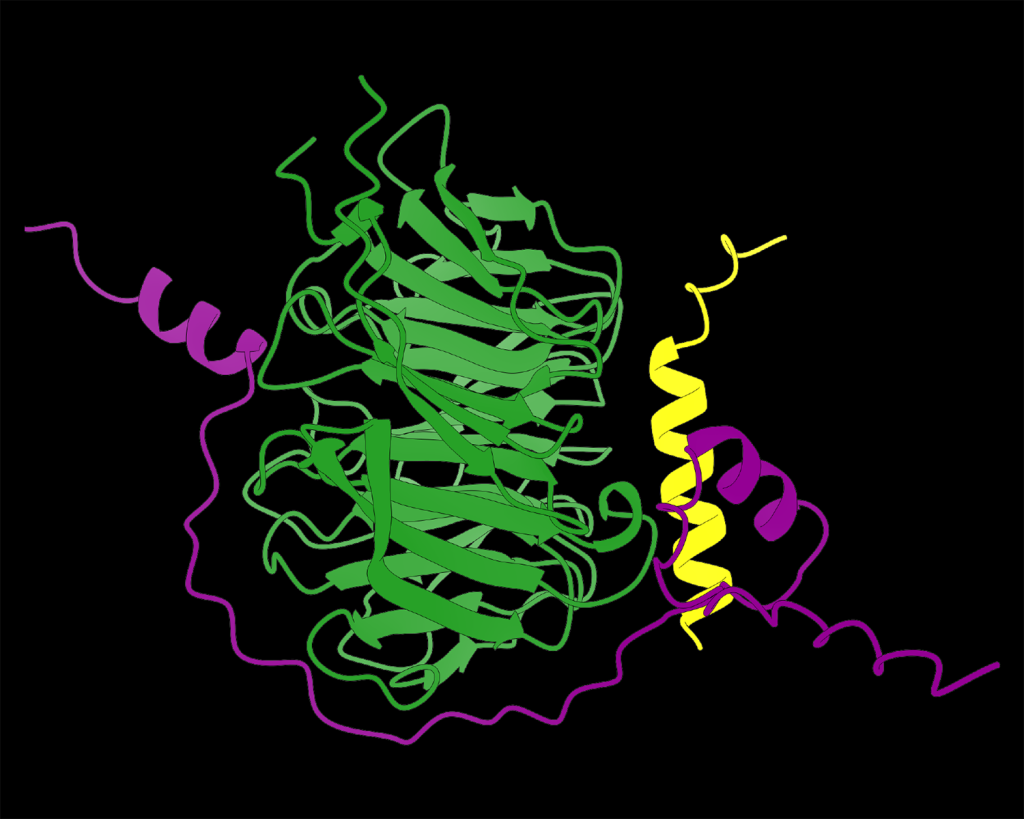
(Courtesy DeBerardinis Lab) AlphaFold structural prediction of PEX39 (purple) binding to PEX7 (green) and a cargo protein (yellow).
The newly-identified PEX39 uncovers a new principle in peroxisomal protein import. PEX39 binds proteins destined for transport to peroxisomes, then passes them to other components of the import machinery, similar to relay runners handing off a baton. Optimal protein import requires just the right amount of PEX39 — too little PEX39 results in an impaired initiation of the transport process, and too much prevents an efficient handoff.
“Scientists identified peroxisomes in the 1950s, but we still do not fully understand how they are assembled and how they operate,” Dr. DeBerardinis said. “This study fills in an important gap by identifying a new peroxin that maximizes import of enzymes required for peroxisomes to carry out their metabolic functions.”
Understanding peroxisomal biology also helps scientists understand the consequences of their dysfunction, which causes inborn errors of metabolism in children and is a factor in aging and neurodegeneration.
“Defects in peroxisomal protein import cause devastating human diseases, such as Zellweger Spectrum Disorders,” Dr. Chen said. “This study represents a substantial finding in peroxisomal biology that opens new avenues of future research and will advance our understanding of peroxisomal disease.”
Dr. DeBerardinis is a Howard Hughes Medical Institute Investigator and directs the Cellular Networks in Cancer Research Program in the Harold C. Simmons Comprehensive Cancer Center at UT Southwestern. He holds the Eugene McDermott Distinguished Chair for the Study of Human Growth and Development and the Philip O’Bryan Montgomery Jr., M.D., Distinguished Chair in Developmental Biology and is a Sowell Family Scholar in Medical Research.
Dr. Chen is a W.W. Caruth Scholar and was recently awarded the prestigious Wellcome Burroughs Fund Career Award for Medical Scientists.
This research is funded by an AAP Marshall Klaus Award, Thrasher Research Fund Early Career Award, Children’s Health Fellow Research Scholar Award, and the Howard Hughes Medical Institute Investigator Program.
About CRI
Children’s Medical Center Research Institute at UT Southwestern (CRI) is a joint venture of UT Southwestern Medical Center and Children’s Medical Center Dallas. CRI’s mission is to perform transformative biomedical research to better understand the biological basis of disease. Located in Dallas, Texas, CRI is home to interdisciplinary groups of scientists and physicians pursuing research at the interface of regenerative medicine, cancer biology, and metabolism — relentless discovery toward the treatments of tomorrow.
X/Twitter | Blue Sky | LinkedIn | Instagram | YouTube | Facebook