Cells within tissues live in a complex mechanical environment in which they must sense and respond to signals. Mechanosensing has recently emerged as a key underlying process that regulates how the immune system functions, stem cells acquire specific fates, and wounds heal. Yet, at a fundamental level, we don’t understand the molecular logic cells use to mechanically sense their environment.
Our laboratory studies cell migration in both immune and cancer cells, which use systems where movement is central to function and pathology. Migration requires the seamless integration of many subroutines: cells must establish a front–back axis, decide where and when to move, and coordinate protrusions at the front and contractions at the rear. What are the rules of this self-organization? How do biochemical signals and mechanical forces collaborate to position polarity programs?
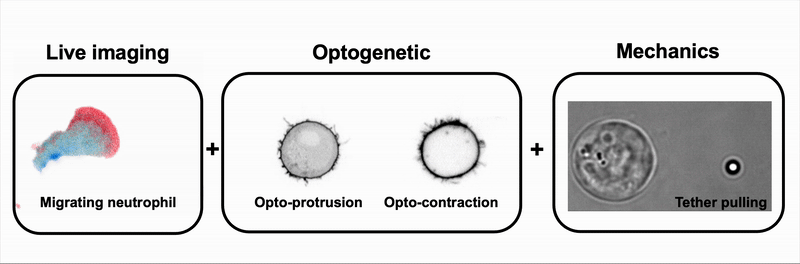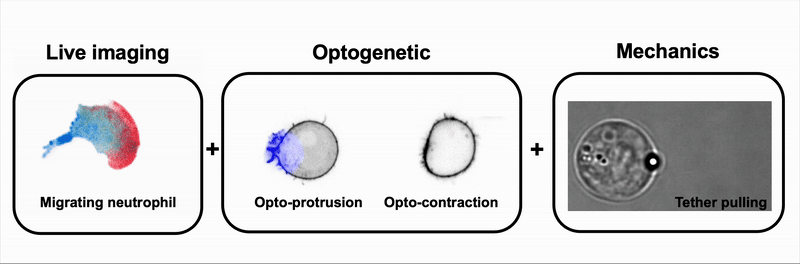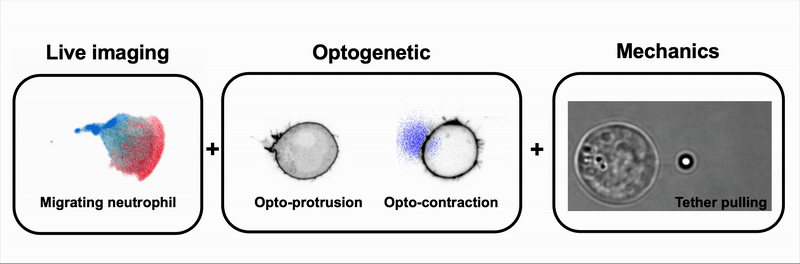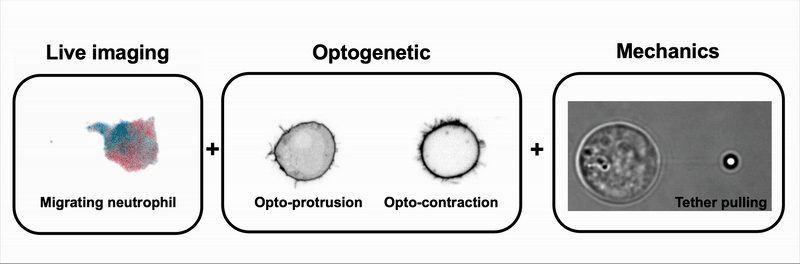
We combine tools to visualize (live imaging), control (optogenetic) and measure (optical tweezers) the interplay between cell signaling and mechanics during immune and cancer cell migration.
Membrane tension is a fundamental physical property that shapes nearly every aspect of cell biology. It coordinates signaling, trafficking, migration, and polarity. To function, cells must keep membrane tension within a narrow optimal range — too high or too low, and essential processes falter. Yet, numerous internal and external factors can acutely alter membrane fluidity and tension. Just as a guitar string must be precisely tuned to play the right note, a cell’s membrane must maintain the right tension to coordinate movement, communication, and essential functions.
Cells, therefore, require robust mechanisms to sustain membrane homeostasis in the face of perturbations. This need is particularly evident in immune cells, whose polarity and motility depend on exquisitely-tuned mechanics, even as they navigate complex and shifting tissue environments. How cells sense and rapidly correct changes in membrane fluidity and tension, however, remains poorly understood.
We aim to uncover how cells preserve membrane homeostasis within complex tissues. We focus on the tumor microenvironment, where metabolic gradients, poor vascularization, and chronic inflammation generate mechanically hostile niches. By examining how immune and tumor cells differentially adapt to these stresses, we seek to identify strategies that selectively reinforce membrane homeostasis, in order to enhance immune cell infiltration while constraining malignant invasion and spread.
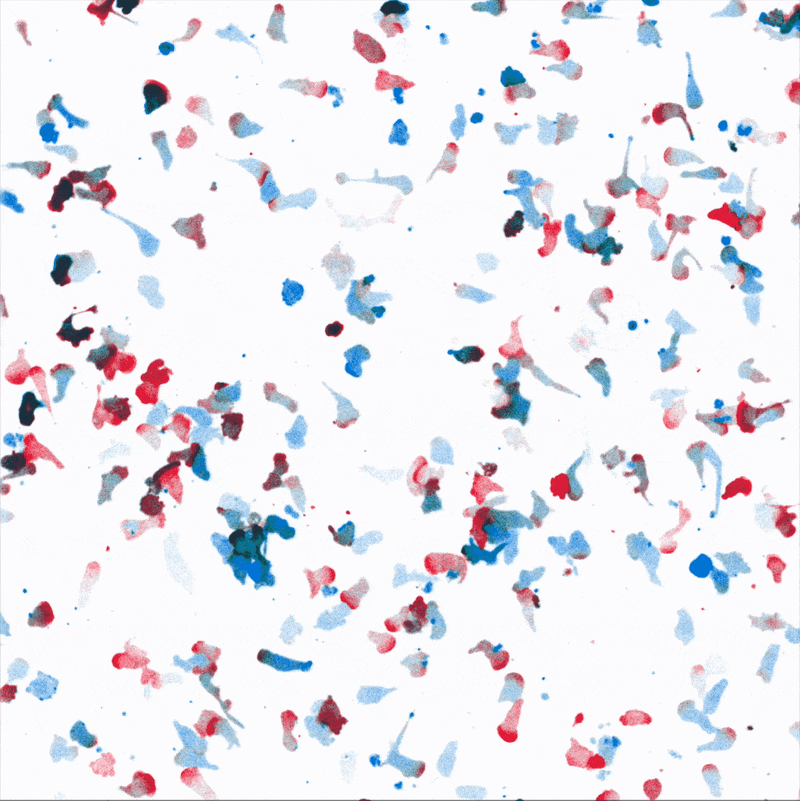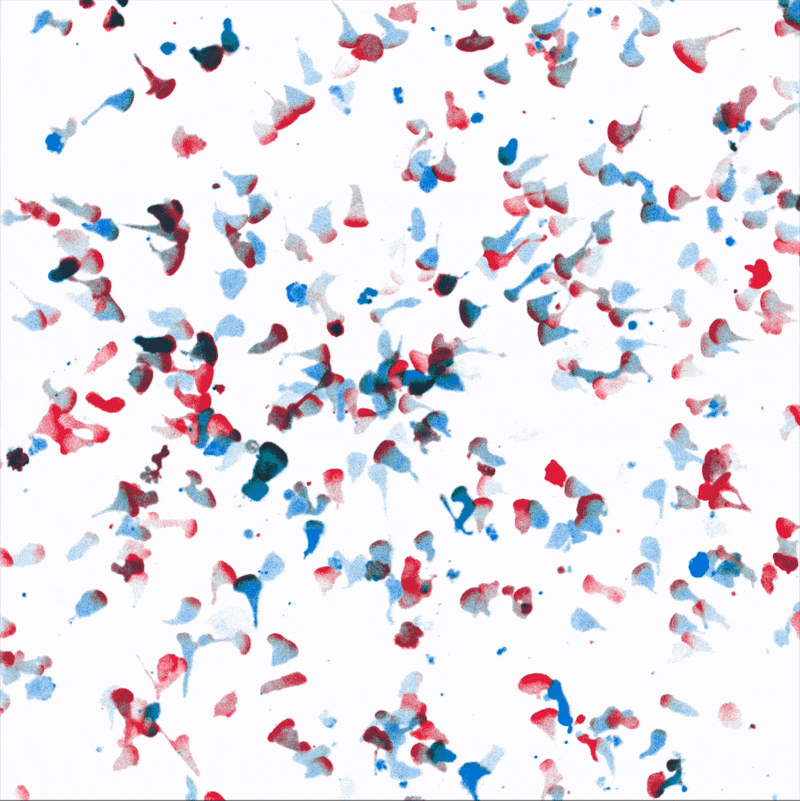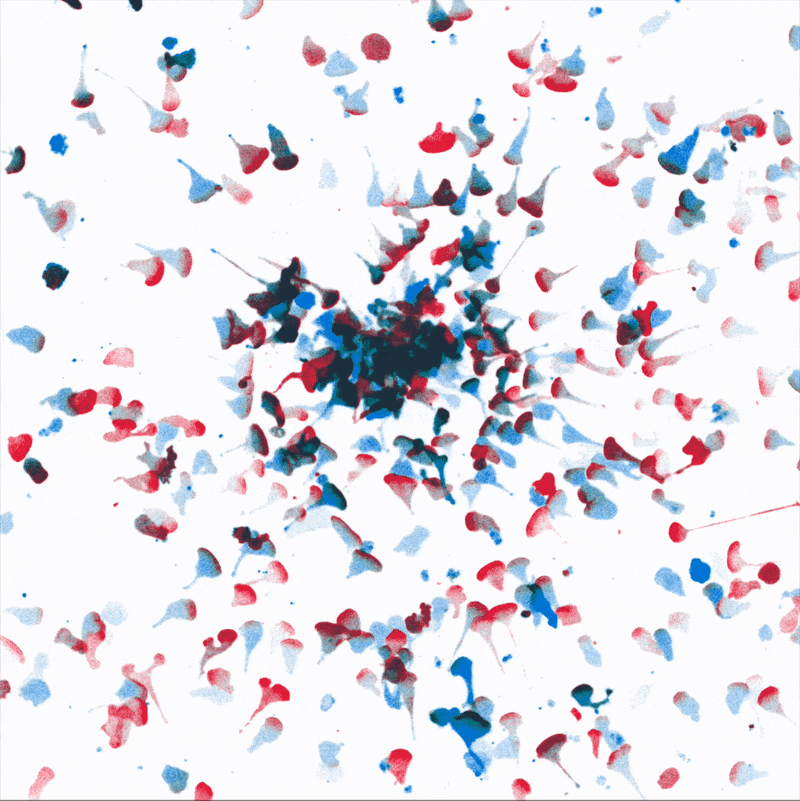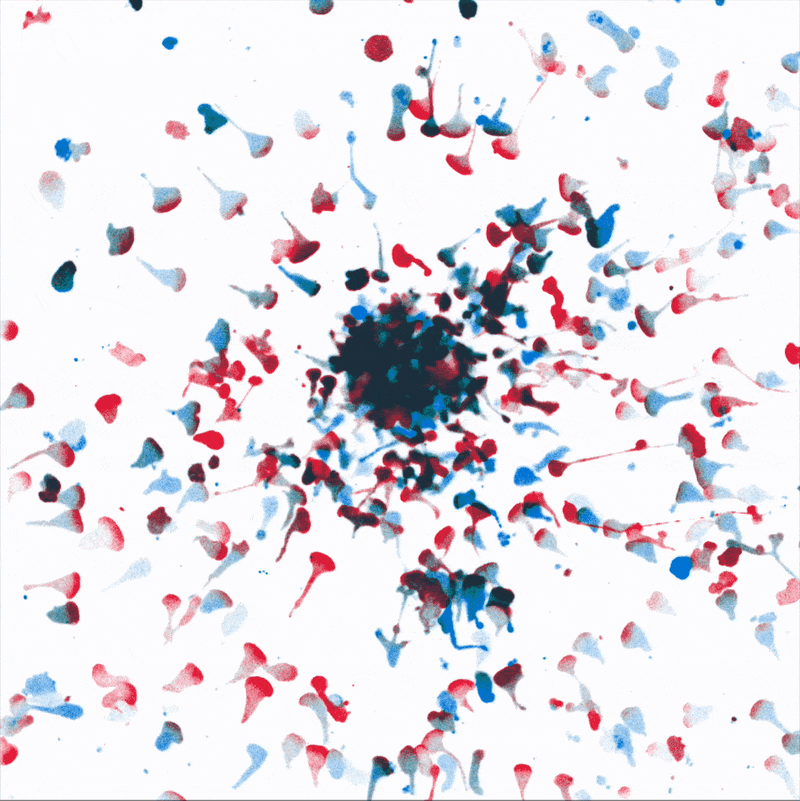
Directional movement of white blood cells to a point source of attractant (center). We are dissecting how environmental stresses influence immune cell’s ability to reach their targets.
Cells rarely act alone. Multicellular coordination enables a dizzying array of cellular processes from the healing of wounds, to the beating of our hearts, and the digestion of our meals. A striking example of this coordination is collective migration, which is when cells move as a cohesive unit rather than as isolated individuals.
Collective migration underlies a wide spectrum of biological processes such as embryonic development and wound repair. In metastasis, tumor cells often leave the primary site as clusters rather than as single cells. Although less abundant, these clusters are strikingly potent, estimated to be 20 to 100 times more efficient at seeding metastases than solitary circulating tumor cells. The ability to polarize as a group appears to be a key determinant of their invasiveness, yet the mechanisms by which they form, polarize, and migrate remain poorly understood.
Our previous work has shown that mechanical signals transmitted within the membrane are essential for single migrating cell polarization and migration. We hypothesize that a similar mechanical-polarity program drives the behavior of cancer clusters. Using optogenetics, mechanical measurements, and migration assays — in both cell lines and patient-derived samples — we aim to uncover the fundamental rules of cluster cohesion and invasion. More broadly, this research asks how cells leverage membrane mechanics to coordinate collective behaviors — principles that span from tissue repair to malignant spread and may reveal new vulnerabilities to halt metastasis at its roots.
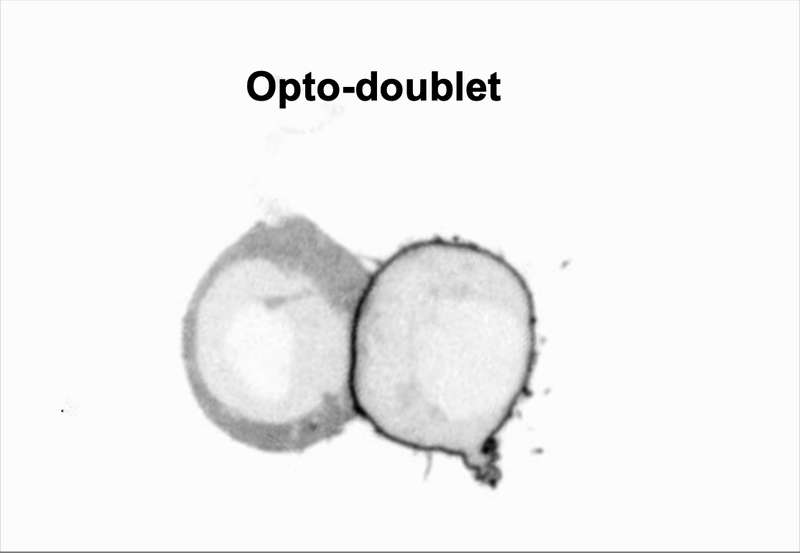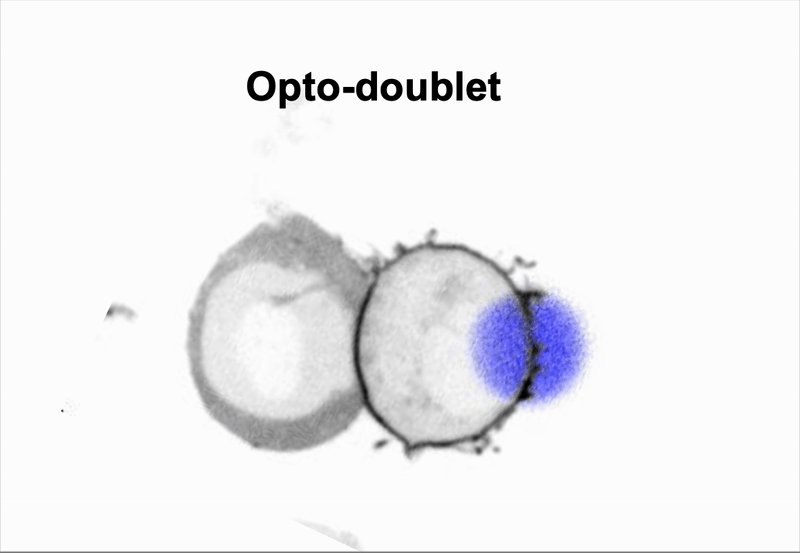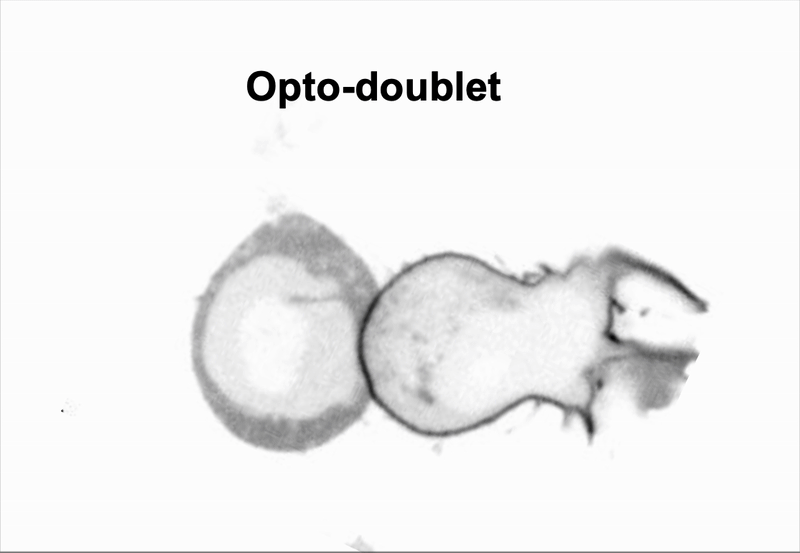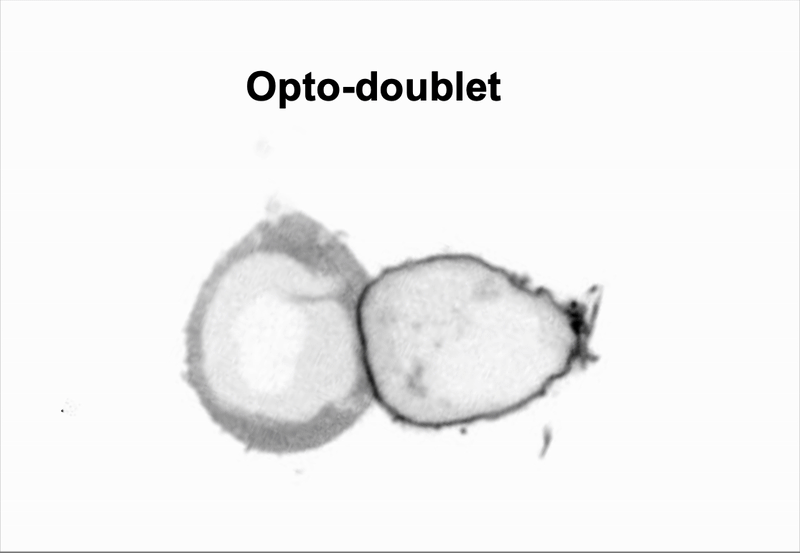
We use doublet of cells to interrogate how cells use mechanics to coordinate multicellular behaviors, such as collective migration encountered during metastasis.






