Peter Ly, Ph.D., is our newest faculty member, transitioning his lab to Children’s Research Institute in fall 2025. Dr. Ly received his bachelor’s in biology from Baylor University and his doctorate in cancer biology from UT Southwestern Medical Center. His graduate work with Dr. Jerry Shay and Dr. Woodring Wright examined how aneuploidy, an abnormal number of chromosomes, contributes to malignant transformation. In 2013, Dr. Ly pursued postdoctoral training at the Ludwig Institute for Cancer Research and University of California San Diego with Dr. Don Cleveland. His postdoctoral research focused on understanding how mitotic chromosome segregation errors drive complex genomic rearrangements through a process called chromothripsis. In 2019, Dr. Ly returned to UT Southwestern to establish his independent group in the Department of Pathology. Now part of CRI, Dr. Ly holds secondary appointments in Cell Biology, Pediatrics, and the Harold C. Simmons Comprehensive Cancer Center at UT Southwestern.
Why did you decide to join CRI?
I was drawn to CRI due to its commitment to bold, curiosity-driven science and sharp focus on uncovering fundamental mechanisms underlying development and disease. The diverse expertise of CRI investigators and its collaborative culture creates an intellectually rich environment that encourages ambitious questions to be asked. I believe this environment will enable and inspire my lab to push the boundaries in understanding how complex genome rearrangements arise during cancer evolution. I am incredibly excited to be part of the CRI community.

What are you researching?
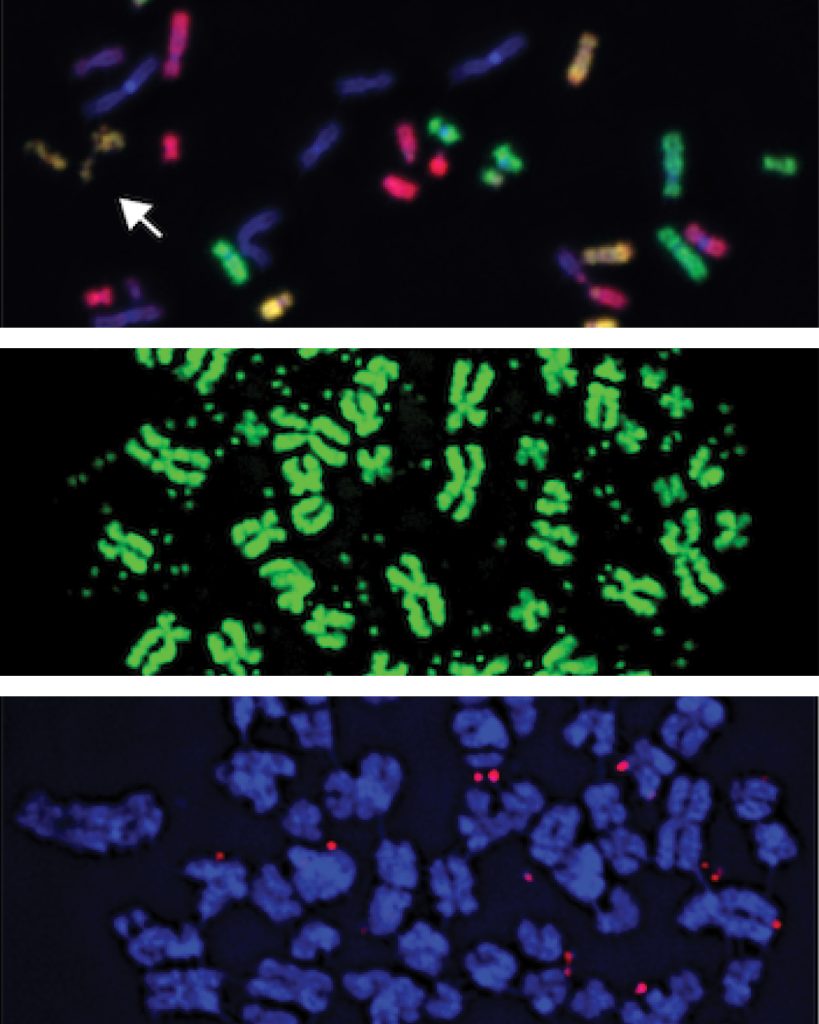
My lab investigates the mechanistic origins of complex alterations in cancer genomes, particularly those caused by errors in mitotic cell division. Every time a cell divides, the duplicated genome must be accurately partitioned between the two daughter cells, but this isn’t always perfect. Some chromosomes can get left behind and become trapped in abnormal structures called micronuclei, which are unstable and prone to DNA damage. We and others have shown that this can trigger a catastrophic process known as chromothripsis, in which a single chromosome shatters into many fragments and then becomes haphazardly stitched back together in the wrong order. The resulting complex rearrangements can delete tumor suppressor genes, generate oncogenic fusions, or amplify genes that drive resistance to therapy. Remarkably, all of these changes can occur simultaneously within just a few cell cycles. Our long-term goal is to understand how mitotic errors, micronuclei, and chromothripsis contribute to genome instability, tumorigenesis, and resistance to anti-cancer therapies.
How do you expect your work will one day help patients?
A fundamental understanding of how cancer genomes evolve is essential for developing strategies to effectively target cancer cells in the clinic. For example, we recently discovered that pathological activation of the Fanconi anemia pathway – a key DNA repair mechanism – can promote chromothripsis and the formation of extrachromosomal DNA (ecDNA) that amplifies genes linked to drug resistance. We then showed that inactivating this pathway can prevent resistance to both chemotherapy and targeted therapies. These proof-of-principle experiments provide strong rationale for advancing our findings into preclinical animal models with the goal of translating them into therapies that will benefit patients.


What led to your career in science?
I was a relatively late bloomer in science. I didn’t grow up playing with microscopes or dreaming of becoming a scientist. My parents escaped Cambodia as refugees at an early age before settling in the United States, so growing up I had limited exposure to what a career in science could even look like. As a first-generation college student, I struggled through introductory biology classes since I was trying to memorize the textbook rather than actually learning the material. Things changed once I started working in the lab, which motivated me to understand how the discoveries I read about in the textbooks were made. After that, my coursework improved, and I began to seriously consider a scientific career and haven’t looked back since. This career path has allowed me the freedom to ask interesting, fundamental questions while contributing to something much bigger than myself: building knowledge that could improve human health and training the next generation of researchers.
What do you like to do when you’re not in the lab?
Outside the lab, my time is devoted to my family. We have three young kids, so our weekends are mostly filled with soccer games, birthday parties, or just chasing them around the house or at the park. I wouldn’t trade it for anything in the world.