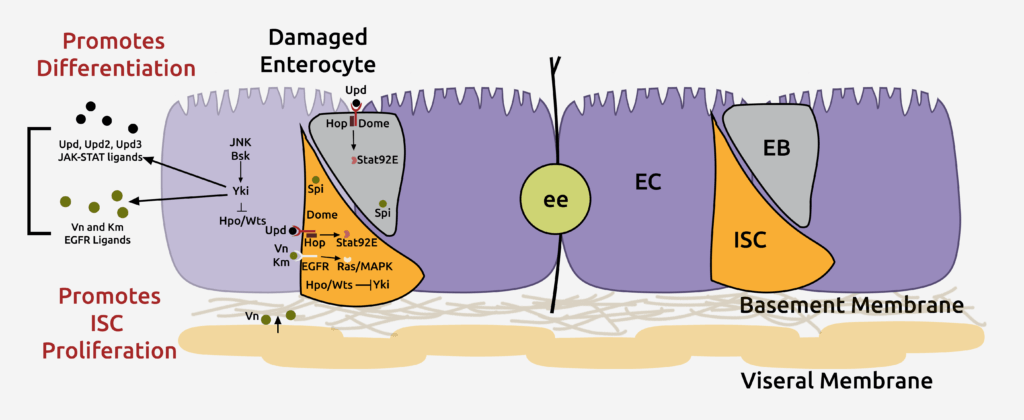
Enterocytes secrete various ligands to promote the proliferation of intestinal stem cells (ISCs) in response to injury, ensuring rapid tissue repair. However, ISCs must return to basal self-renewal rates to avoid overgrowth after tissue repair. Currently, the mechanisms that prevent hyperplasia following injury remain largely unknown.
Our published findings indicate that the bone morphogenetic protein (BMP) signaling pathway is activated in response to injury to suppress ISC proliferation. These findings also provide evidence for how co-regulation of antagonistic signals mediates tissue homeostasis and how disconnect between these signals can lead to abnormal tissue homeostasis (Journal of Cell Biology 201, 945-61, 2013). Indeed, loss-of-function mutations in the BMP receptor type 1A and mothers against decapentaplegic homolog 4 (SMAD4s) have been found in human gastrointestinal polyposis syndromes, underscoring the importance of the effect of BMP-signaling on proliferation. The BMP-signaling pathway functions in the ISCs to antagonize ISC proliferation following injury. However, the transcriptional downstream targets of BMP signaling remain unknown.
We are currently testing phosphatase and tensin homolog (PTEN) and Wnt signaling as candidate downstream targets of BMP signaling, as they have been established as targets of BMP signaling in the mammalian intestine. In parallel, we have performed RNA-seq analysis between wild-type and BMP mutant intestines to identify novel evolutionarily conserved downstream targets of BMP-signaling and are currently analyzing the results in both fly and mouse tissues. Given the similarities between Drosophila and mammalian intestinal homeostasis, identifying the mechanism by which BMP-signaling regulates ISC proliferation in our model system will presumably have broad clinical implications for the diagnosis and treatment of colon cancer.
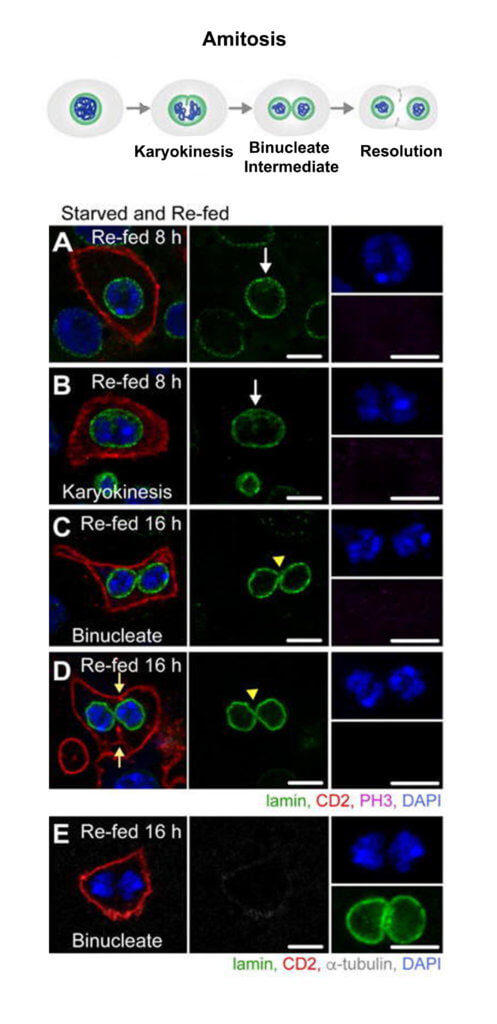
Studies of intestinal homeostasis have focused largely on replacement of differentiated cells that are lost due to digestive function and exposure to ingested bacteria and chemical damage. However, mechanisms of ISC replacement have been largely unexplored. To determine if and how Drosophila ISCs are replaced after loss, we developed a starvation assay to induce rapid loss of stem cells. Within two days of starvation, severe regional loss of approximately half of the total ISC population in the posterior midgut occurred. Upon re-feeding of starved animals, the ISC number returned to that of controls, demonstrating that mechanisms, currently unknown, are in place to precisely regulate the ISC number. Studies in other systems have revealed that lost stem cells are replaced by symmetric divisions of remaining stem cells. However, our analysis demonstrated that generation of new stem cells appeared to proceed through a spindle-independent ploidy reduction of cells in the enterocyte lineage through a process known as amitosis (Cell Stem Cell 20, 609-620, 2017). Amitosis was first recognized as a specialized form of cell division in chicken red blood cells by Robert Remak in 1841 and has been identified in a vast array of species, from primitive ciliates to mammals, and is implicated in generation of some forms of cancer.
Our work establishes a new paradigm of stem cell replacement involving dedifferentiation of polyploid enterocytes to ISCs and may provide insight into the currently unknown mechanisms of ploidy reduction in tissue homeostasis and cancer. Ploidy reduction has been documented in hepatocytes, and ploidy reduction of tumor cells that escape mitotic catastrophe secondary to irradiation has been shown to give rise to a mitotically active population of cells. In addition, we have determined that amitosis initiates in aging animals and upon ISC dysfunction during proliferative demand. As we now have multiple assays that induce ploidy reduction of enterocytes, we can use this model to identify pathways that regulate this enigmatic process and to provide mechanistic insight into amitosis and ploidy reduction in other systems.
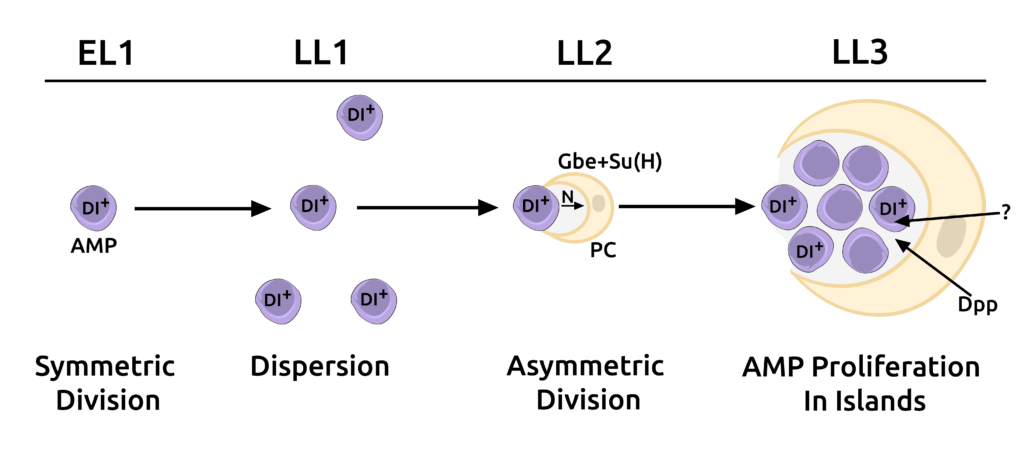
Stem cell niches provide microenvironments for stem cell proliferation and maintenance. However, even though the role of niches in the maintenance of tissue homeostasis has been well examined, relatively little is known about their function in establishing stem cell lineages during organogenesis. The cells of the adult Drosophila midgut are generated during larval development from adult midgut progenitors (AMPs). Our lab has identified a novel cell, the peripheral cell (PC), in the Drosophila larval midgut that is generated via Notch signaling by AMPs. The PC signals via BMP and other unidentified pathways to repress AMP differentiation, thereby acting as a niche. We found that this niche provides a holding pen for AMPs to proliferate in an undifferentiated manner during development but then breaks down and loses contact with AMPs during metamorphosis to allow cells of the adult midgut, including ISCs to be established (Science 327, 210-3, 2010).
Overall, our work presents a new paradigm where a stem cell can generate its own niche. The ability of a stem cell to generate its own niche lends greater autonomy to stem cells to both regulate their numbers and to vary their locations in a tissue without the constraints of a fixed niche. This ability has important implications in maintaining homeostasis and tissue patterning as well as in cancer biology, where cancerous cells exhibit stem cell-like properties. Using a combination of genetic and cell biological approaches, we are attempting to identify other cellular and molecular mechanisms that explain how PCs regulate stem cell maintenance and enterocyte differentiation.