Javier Garcia-Bermudez, Ph.D.
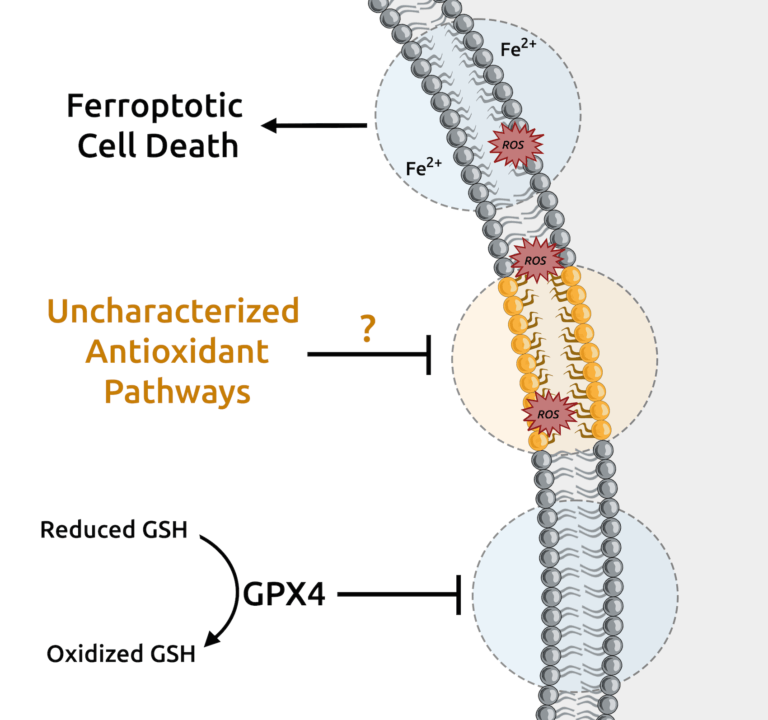
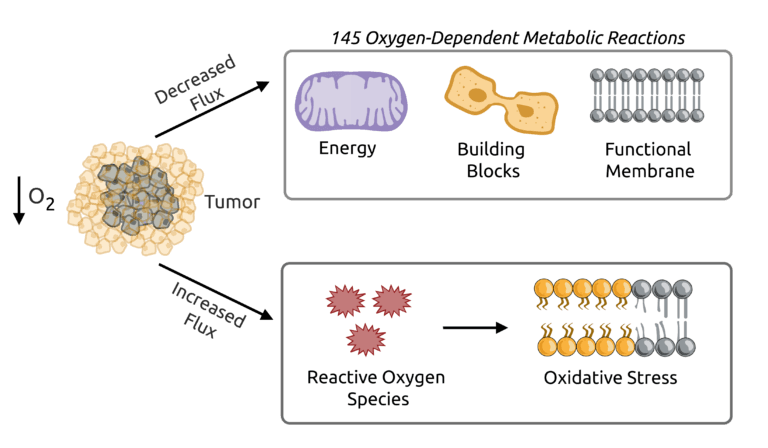
Javier Garcia-Bermudez received his bachelor’s degree in biochemistry from the Autonomous University of Madrid in Spain. He then went on to obtain his Ph.D. in biochemistry and molecular biology from the same university, studying the role of mitochondrial respiration in cancer progression and stem cell differentiation. In 2016, Dr. Garcia-Bermudez joined Dr. Kivanc Birsoy’s laboratory at Rockefeller University as a European Molecular Biology Organization Long-Term Fellow and as a Leukemia and Lymphoma Society Special Fellow. His postdoctoral work uncovered critical metabolic dependencies of cancers exposed to low oxygen and lipid peroxidation stress.
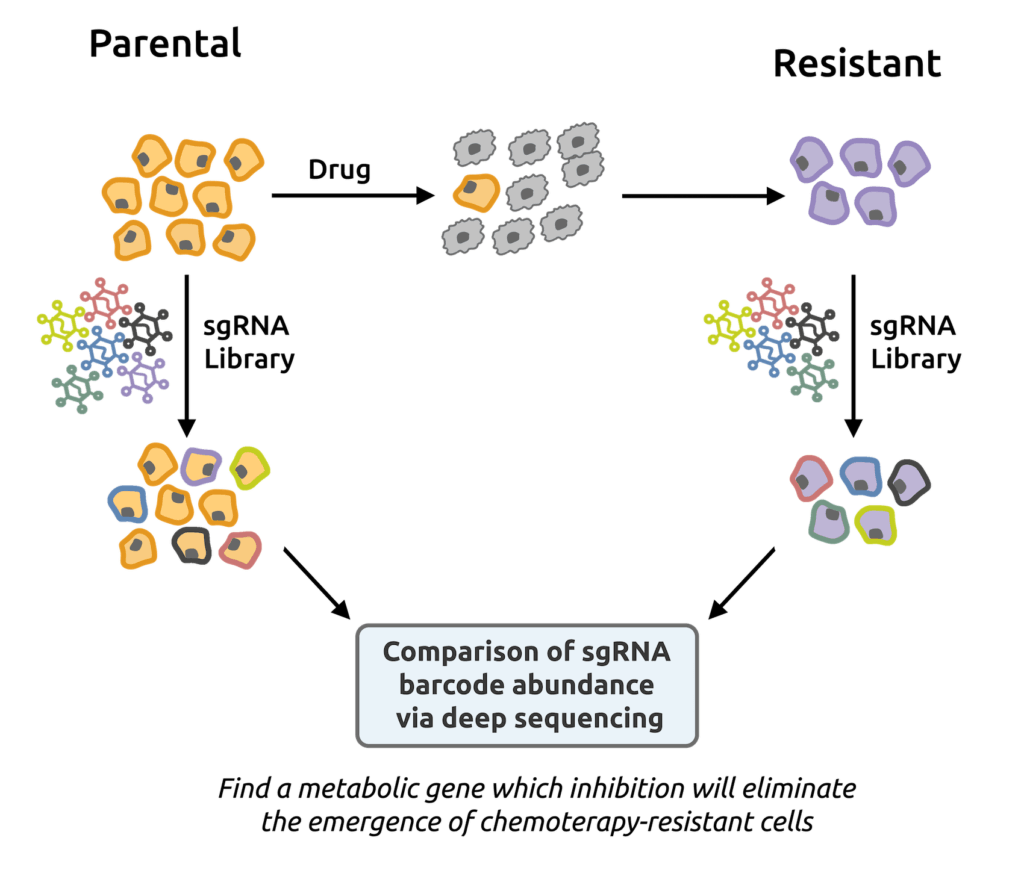
Dr. Garcia-Bermudez joined the faculty of Children’s Medical Center Research Institute at UT Southwestern in December 2021. He is a Cancer Prevention and Research Institute of Texas Scholar and has received a transition award from the National Cancer Institute (NCI-NIH) to support his studies on metabolic liabilities of tumors.