Shelton, S.D., House, S., Nascentes Melo, L.M., Ramesh, V., Chen, Z., Wei, T., Wang, X., Llamas, C.B., Krishna Venigalla, S.S., Menezes, C.J., Allies, G., Krystkiewicz, J., Rosler, J., Meckelmann, S.W., Zhao, P., Rambow, F., Schadendorf, D., Zhao, Z., Gill, J.G., DeBerardinis, R.J., Morrison, S.J., Tasdogan, A., and P. Misrha. (2024). Pathogenic mitochondrial DNA mutations inhibit melanoma metastasis. Sciences Advances 10:eadk8801. (Link)
Xun, W., Menezes, C., Jia, Y., Xiao, Y., Krishna Venigalla, S.S., Cai, F., Hsieh, M.H., Gu, W., Du, L., Sudderth, J., Kim, D., Shelton, S.D., Llamas, C.B., Lin, Y.H., Zhu, M., Merchant, S., Bezwada, D., Kelekar, S., Zacharias, L.G., Mathews, T.P. Hoxhaj, G., Wynn, R.M., Tambar, U.K., DeBerardinis, R.J., Zhu, H., and P. Mishra. (2024). Metabolic inflexibility promotes mitochondrial health during liver regeneration. Science 384:eadj4301. (PubMed)
Wang, X., and
P. Mishra. (2023). Fusion of dysfunction muscle stem cells with myofibers induces sarcopenia in mice.
bioRxiv 10.1101/2023.01.20.524967. (
PubMed)
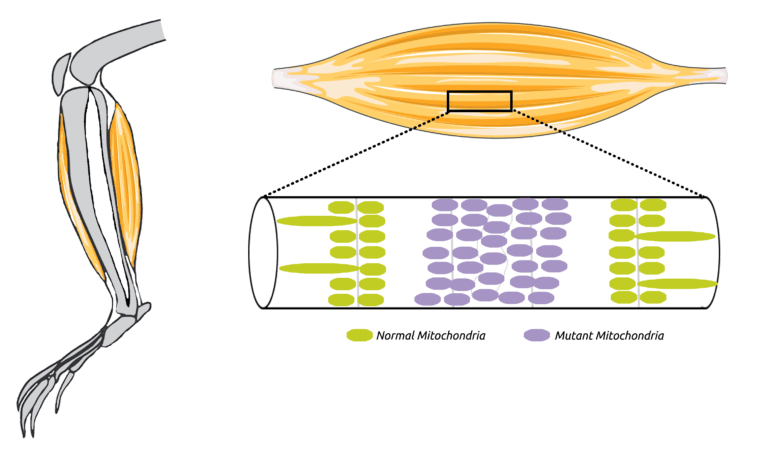
Chen, Z., Bordieanu, B., Kesavan, R., Lesner, N.P., Venigalla, S.S.K., Shelton, S.D., DeBerardinis, R.J., and P. Mishra. (2023). Lactate metabolism is essential in early-onset mitochondrial myopathy. Science Advances 9:eadd3216. (PubMed)
Garg, A., Keng, W.T., Chen, Z., Sathe, A.A., Xing, C., Kailasam, P.D., Shao, Y., Lesner, N.P., Llamas, C.B., Agarwal, A.K., and P. Mishra. (2022). Autosomal recessive progeroid syndrome due to homozygosity for a TOMM7 variant. Journal of Clinical Investigation 132:e156864. (PubMed)
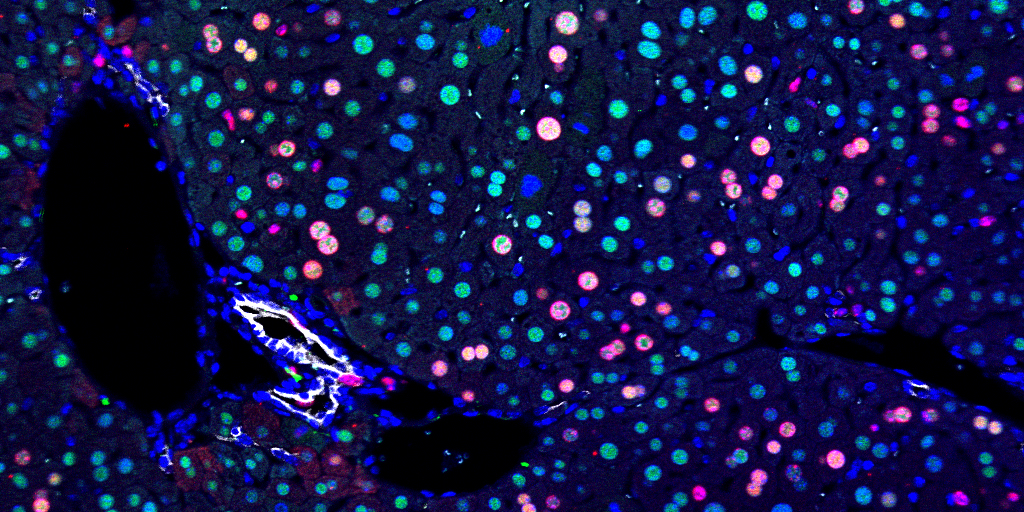
Lesner, N.P., Wang, X., Chen, Z., Frank, A., Menezes, C., House, S., Shelton, S.D., Lemoff, A., McFadden, D.G., Wanaspura, J., DeBerardinis, R.J., and P. Mishra. (2022). Differential requirements for mitochondrial electron transport chain components in the adult murine liver. eLife 11:e80919. (PubMed)
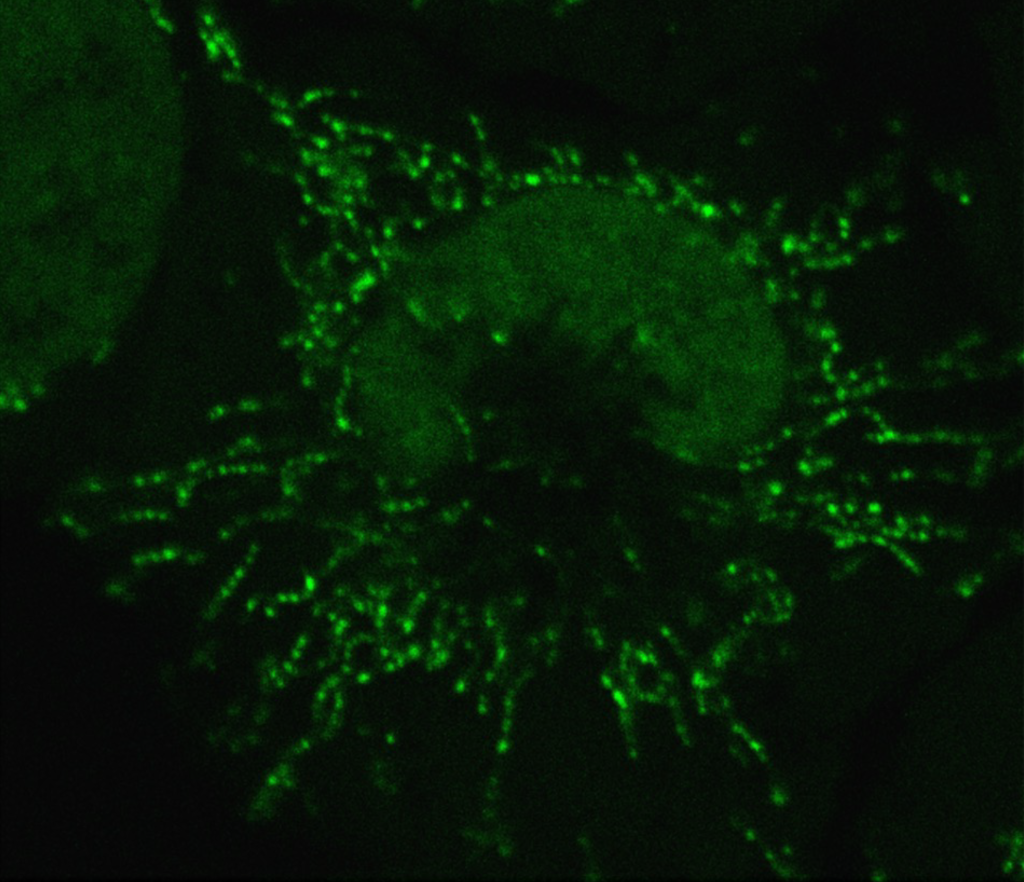
Wang, X., Jia, Y., Zhao, J., Lesner, N.P., Menezes, C.J., Shelton, S.D., Venigalla, S.S.K., Xu, J., Cai, C., and P. Mishra. (2022). A Mitofusin 2 – Hif1α axis sets a maturation checkpoint in regenerating skeletal muscle. Journal of Clinical Investigation 132:e161638. (PubMed)
Wang, X., Shelton, S.D., Bordieanu, B., Frank, A.R., Yi, Y., Venigalla, S.S.K., Gu, Z., Lenser, N.P., Glogauer, M., Chandel, N.S., Zhao, H., Zhao, Z., McFadden, D.G., and P. Mishra. (2022). Scinderin promotes fusion of electron transport chain dysfunctional muscle stem cells with myofibers. Nature Aging 2, 155-169. (PubMed)
Lesner, N.P., Gokhale, A., Kota, K., DeBerardinis, R.J., and P. Mishra. (2020). α-ketobutyrate links alterations in cystine metabolism to glucose oxidation in mtDNA mutant cells. Metabolic Engineering 60, 157-167. (PubMed)
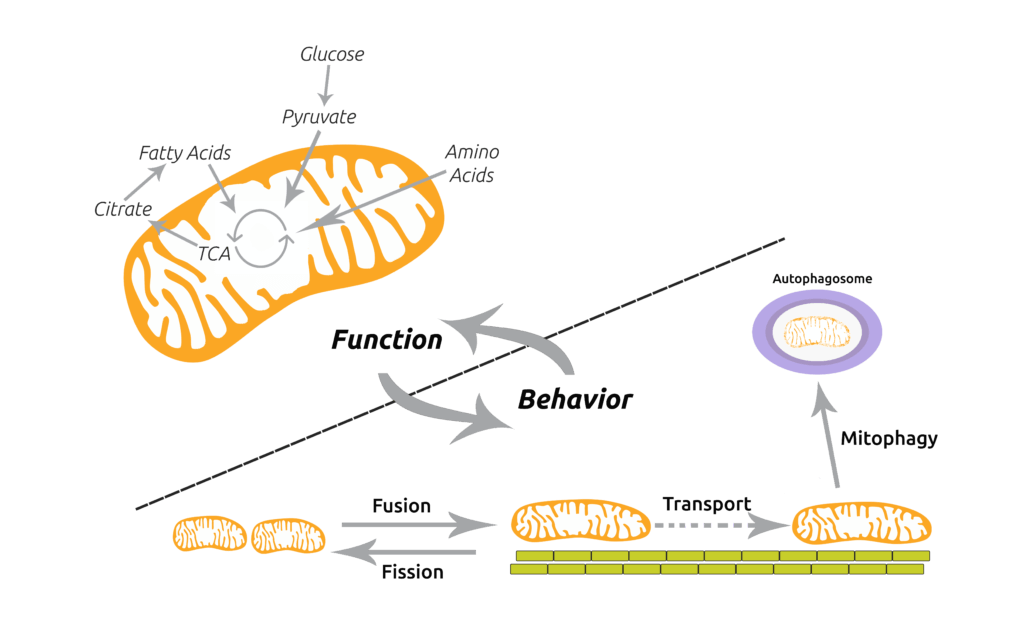
Mishra, P. (2016). Interfaces between mitochondrial dynamics and disease. Cell Calcium 60, 190-8. (PubMed)
Mishra, P., and D.C. Chan. (2016). Metabolic regulation of mitochondrial dynamics. Journal of Cell Biology 212, 379-87. (PubMed)
Mishra, P.,* Varuzhanyan, G.,* Pham, A.H., and D.C. Chan. (2015). Mitochondrial dynamics is a distinguishing feature of skeletal muscle fiber types and regulates organellar compartmentalization. Cell Metabolism 22, 1033-44. (PubMed)
Mishra, P., and D.C. Chan. (2014). Mitochondrial dynamics and inheritance during cell division, development and disease. Nature Reviews Molecular Cell Biology 15, 634-646. (PubMed)
*Contributed equally