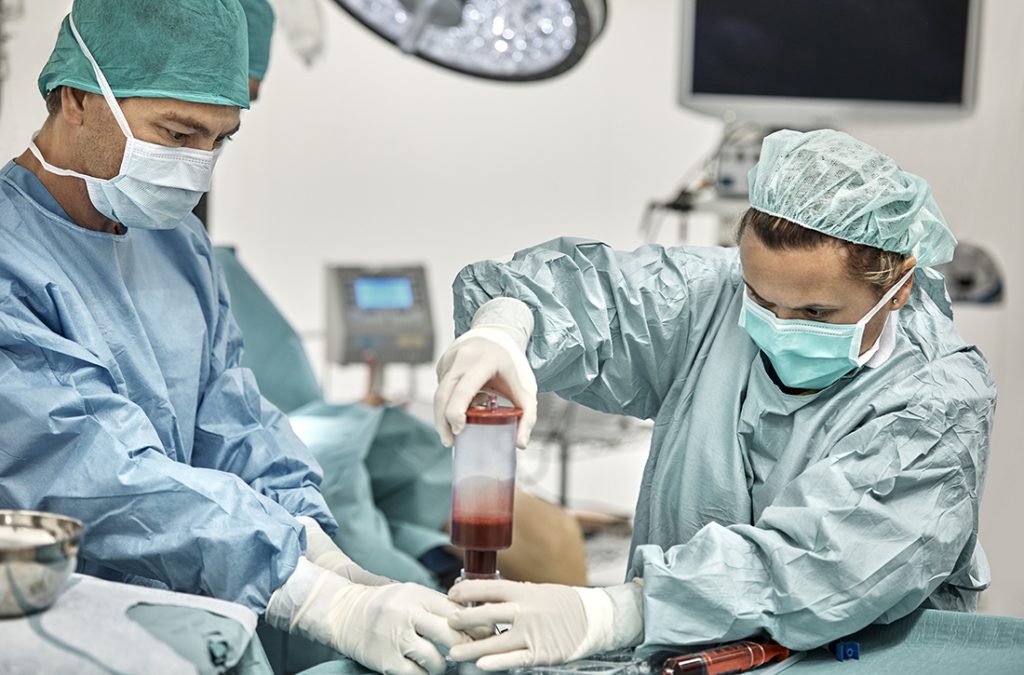
 We study the cell-intrinsic and cell-extrinsic mechanisms that regulate stem cell self-renewal and the roles these mechanisms play in tissue homeostasis and cancer. The maintenance of many adult tissues depends on the persistence of stem cells throughout life. Stem cells are maintained in adult tissues by self-renewal, the process by which stem cells divide to make more stem cells. By better understanding this process, we gain insights into how tissues develop and regenerate, how reduced self-renewal can lead to degenerative disease, and how increased self-renewal can lead to tumorigenesis. We identified a series of mechanisms that distinguish stem cell self-renewal from restricted progenitor proliferation as well as self-renewal mechanisms that are conserved among stem cells in different tissues. We are currently focused on areas of cellular physiology that are understudied in stem cells, including metabolism and proteostasis.
We study the cell-intrinsic and cell-extrinsic mechanisms that regulate stem cell self-renewal and the roles these mechanisms play in tissue homeostasis and cancer. The maintenance of many adult tissues depends on the persistence of stem cells throughout life. Stem cells are maintained in adult tissues by self-renewal, the process by which stem cells divide to make more stem cells. By better understanding this process, we gain insights into how tissues develop and regenerate, how reduced self-renewal can lead to degenerative disease, and how increased self-renewal can lead to tumorigenesis. We identified a series of mechanisms that distinguish stem cell self-renewal from restricted progenitor proliferation as well as self-renewal mechanisms that are conserved among stem cells in different tissues. We are currently focused on areas of cellular physiology that are understudied in stem cells, including metabolism and proteostasis.
We focus on hematopoietic stem cells (HSCs) and the specialized microenvironments, or niches, in which they reside. We identified the location and cellular composition of the HSC niche in bone marrow, including the Leptin Receptor-expressing (LepR+) stromal cells that are the major source of factors for HSC maintenance. These LepR+ cells also include the skeletal stem cells that are the main source of new osteoblasts and adipocytes in adult bone marrow. We discovered a new bone-forming growth factor, Osteolecin, that is produced by the LepR+ cells and that promotes the maintenance and repair of the adult skeleton. LepR+ cells also give rise to osteogenic and adipogenic progenitors that create niches for hematopoietic stem and progenitor cells. We also identified the HSC niche for extramedullary hematopoiesis in the spleen that is activated when the bone marrow is damaged by chemotherapy, radiation, or cancer. This is an exciting time in niche biology as we now have the possibility of going beyond growth factors to ask what other mechanisms niches use to regulate stem and progenitor cell function. Recent studies from our lab have identified metabolic and mechanical regulation.
 Much of age-related morbidity in mammals may be determined by the influence of aging on stem cell function. We have found that stem cells from the hematopoietic and nervous systems undergo strikingly conserved changes in their properties as they age, including declining self-renewal capacity.
Much of age-related morbidity in mammals may be determined by the influence of aging on stem cell function. We have found that stem cells from the hematopoietic and nervous systems undergo strikingly conserved changes in their properties as they age, including declining self-renewal capacity.
We have identified a network of heterochronic gene products that regulates stem cell maintenance throughout life while also regulating the temporal changes in stem cell properties required to match the changing growth and regeneration demands of fetal and adult tissues. Proto-oncogenic signals dominate during fetal development when tissue growth is rapid but cancer risk is low, and tumor-suppressor mechanisms are amplified during aging when there is little tissue growth but cancer risk is high. The increase in tumor suppressor expression during aging delays the development of cancer but also impairs stem cell function and tissue regenerative capacity.
 Cancer cells hijack stem cell self-renewal mechanisms by acquiring mutations that over-activate these pathways. By comparing the mechanisms that regulate the self-renewal of normal stem cells and the self-replication of cancer cells, we identify differences that represent potential vulnerabilities that can be targeted to kill cancer cells.
Cancer cells hijack stem cell self-renewal mechanisms by acquiring mutations that over-activate these pathways. By comparing the mechanisms that regulate the self-renewal of normal stem cells and the self-replication of cancer cells, we identify differences that represent potential vulnerabilities that can be targeted to kill cancer cells.
We are particularly interested in the mechanisms that regulate melanoma metastasis. We discovered that the survival of melanoma cells during metastasis is limited by oxidative stress. The rare cells that survive metastasis appear to undergo metabolic changes that enhance oxidative stress resistance. By better understanding these mechanisms, we hope to develop pro-oxidant therapies that inhibit cancer progression by exacerbating the oxidative stress experienced by cancer cells.
Cancer cells, including melanoma, often metastasize regionally through lymphatics before metastasizing systemically through the blood. We found that melanoma cells in lymph experience less oxidative stress and form more metastases than melanoma cells in the blood. This is true in both patient-derived melanomas in immunocompromised mice and mouse melanomas in syngeneic, immunocompetent mice. Cells metastasizing through blood, but not lymph, appear to undergo ferroptosis, a form of cell death marked by lipid oxidation. Multiple differences between lymph and blood may contribute to this difference in oxidative stress, including higher levels of oleic acid in lymph. Oleic acid is a monounsaturated fatty acid that can protect cancer cells from ferroptosis by reducing the abundance of oxidizable polyunsaturated fatty acids in phospholipids. We found it is incorporated by melanoma cells in lymph and promotes their survival in the blood. This offers a potential explanation for a clinical behavior that is the basis for much of cancer staging and treatment—the tendency of melanomas and epithelial cancers to metastasize first through lymphatics and then through the blood.