 Children’s Medical Center Research Institute at UT Southwestern (CRI) has identified a biomarker that enables researchers to accurately characterize the properties and function of mesenchymal stem cells (MSCs) as they exist in the body. MSCs are the focus of nearly 200 active clinical trials registered with the U.S. National Institutes of Health, targeting conditions such as bone fractures, cartilage injury, degenerative disc disease and osteoarthritis.
Children’s Medical Center Research Institute at UT Southwestern (CRI) has identified a biomarker that enables researchers to accurately characterize the properties and function of mesenchymal stem cells (MSCs) as they exist in the body. MSCs are the focus of nearly 200 active clinical trials registered with the U.S. National Institutes of Health, targeting conditions such as bone fractures, cartilage injury, degenerative disc disease and osteoarthritis.
The finding, published in Cell Stem Cell, significantly advances the field of MSC biology, and if the same biomarker identified in CRI’s studies with mice works in humans, the outlook for clinical trials that use MSCs will be improved by the ability to better identify and characterize the relevant cells.
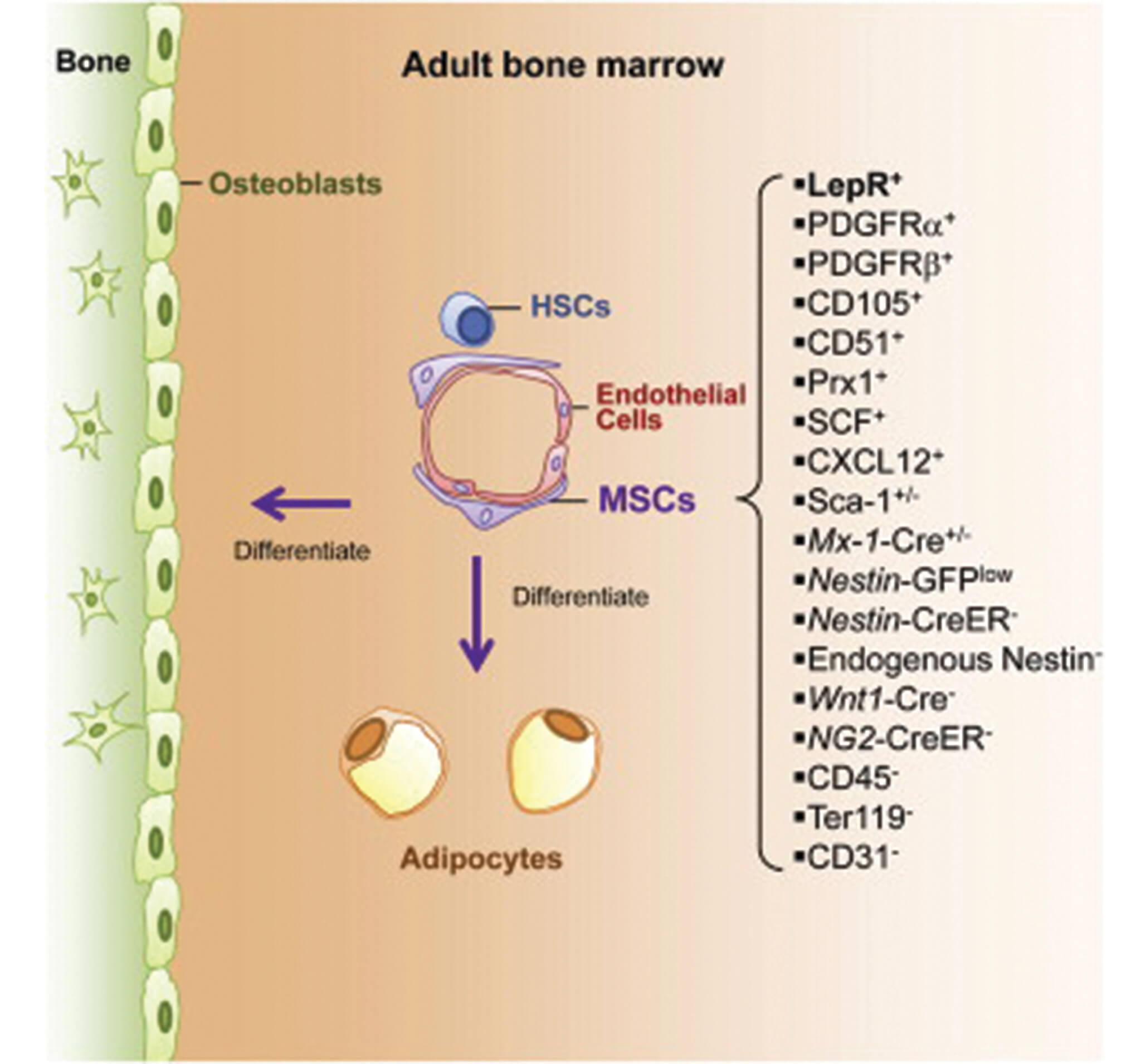
“There has been an increasing amount of clinical interest in MSCs, but advances have been slow because researchers to date have been unable to identify MSCs and study their normal physiological function in the body,” said Dr. Sean Morrison, Director of the Children’s Research Institute, Professor of Pediatrics at UT Southwestern Medical Center, and a Howard Hughes Medical Institute Investigator. “We found that a protein known as Leptin Receptor can serve as a biomarker to accurately identify MSCs in adult bone marrow in vivo, and that those MSCs are the primary source of new bone formation and bone repair after injury.”
In the course of their investigation, the CRI researchers found that Leptin Receptor-positive MSCs are also the main source of factors that promote the maintenance of blood-forming stem cells in the bone marrow.
“Unfortunately, many clinical trials that are testing potential therapies using MSCs have been hampered by the use of poorly characterized and impure collections of cultured cells,” said Dr. Morrison, senior author of the study and holder of the Mary McDermott Cook Chair in Pediatric Genetics at UT Southwestern. “If this finding is duplicated in our studies with human MSCs, it will improve the characterization of MSCs that are used clinically and could increase the probability of success for well-designed clinical trials using MSCs.”
Dr. Bo Zhou, a Leukemia & Lymphoma Society Fellow in Dr. Morrison’s laboratory, was first author of the paper. Other CRI researchers involved in the study were Dr. Rui Yue, a Damon Runyon Fellow, and Dr. Malea Murphy, a postdoctoral research fellow. The project was supported by the National Heart, Lung and Blood Institute, the Cancer Prevention and Research Institute of Texas, and donors to the Children’s Medical Center Foundation.
About CRI
Children’s Medical Center Research Institute at UT Southwestern (CRI) is a joint venture established in 2011 to build upon the comprehensive clinical expertise of Children’s Medical Center of Dallas and the internationally recognized scientific excellence of UT Southwestern Medical Center. CRI’s mission is to perform transformative biomedical research to better understand the biological basis of disease, seeking breakthroughs that can change scientific fields and yield new strategies for treating disease. Located in Dallas, Texas, CRI is creating interdisciplinary groups of exceptional scientists and physicians to pursue research at the interface of regenerative medicine, cancer biology and metabolism, fields that hold uncommon potential for advancing science and medicine. More information about CRI is available on its website: cri.utsw.edu..