Scientists at the Children’s Medical Center Research Institute at UT Southwestern (CRI) have discovered that an aggressive subset of non-small-cell lung cancer (NSCLC) relies on an unusual metabolic pathway to survive. These findings could result in new ways to treat lung cancer, the leading cause of cancer-related deaths worldwide.
Treatment for NSCLC, which accounts for nearly 85 percent of all lung cancers, has evolved as we have identified specific, targetable oncogenes that contribute to tumor growth and progression. Chemotherapy cannot currently inhibit KRAS, one of the most commonly mutated oncogenes in lung cancer. When KRAS mutations occur with the loss of the tumor suppressor LKB1, the resulting tumors are highly aggressive, making this combination of mutations a high priority for new therapies.
Recognizing that mutations in either KRAS or LKB1 resulted in metabolic changes, researchers from the DeBerardinis lab in the CRI set out to identify metabolic changes occurring only when both genes mutated in the same tumor or tumor cells. They found that this combination of mutations strikingly altered the way the tumors handled nitrogen, an essential chemical component of proteins and DNA. In particular, mutations in LKB1 allowed the cells to use an enzyme called CPS1 to transfer nitrogen to DNA. Surprisingly, the usual role of this enzyme in humans to feed a totally different pathway called the urea cycle, which allows excess nitrogen to be excreted from the body. But in cancer cells, CPS1 recaptured nitrogen to supply DNA synthesis, which tumors need to grow.
Depletion of CSP1 in KRAS- and LKB1-mutant tumor cells resulted in defective DNA synthesis, cell death and reduced tumor growth. Tumor growth reduction was even more striking when CPS1 depletion combined with the DNA-damaging agent cisplatin, a common treatment for NSCLC.
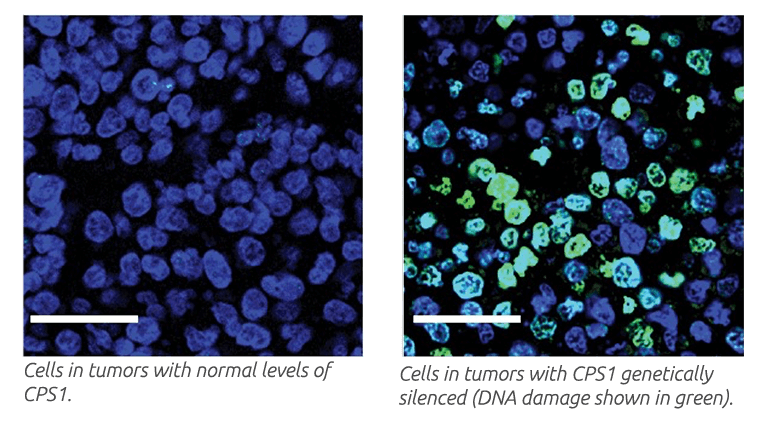
Overall, the findings reveal a new strategy for cancer cells to generate DNA and other building blocks requiring nitrogen. The findings also suggest that components of the CPS1-stimulated pathway may be therapeutic targets in patients with KRAS- and LKB1-mutant NSCLC.
The study is available in Nature.