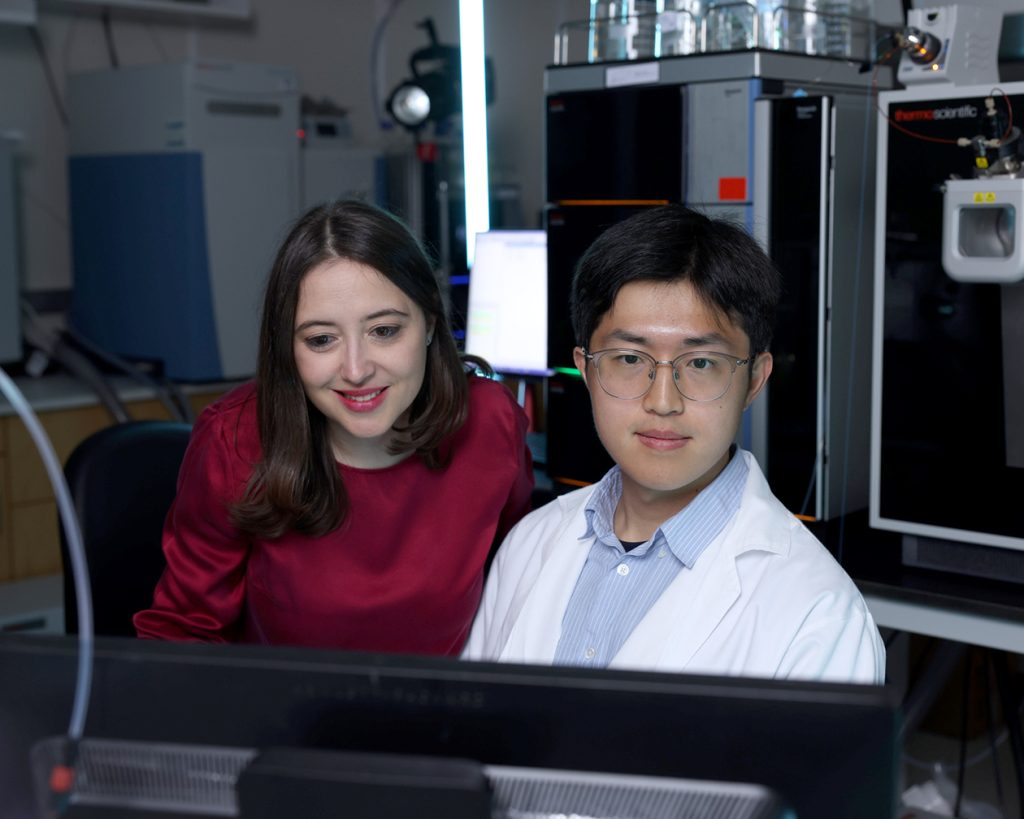
CRI researchers Ralph DeBerardinis, M.D., Ph.D., and Zheng Wu, Ph.D.
Scientists have identified a new way to disrupt lung cancer growth in research from Children’s Medical Center Research Institute at UT Southwestern (CRI) published in Cell Metabolism. Their work illustrates how blockade of the mitochondrial electron transport chain (ETC) alters the way tumors produce purines — building blocks needed to synthesize DNA and RNA — and exposes a metabolic vulnerability in some tumors.
Zheng Wu, Ph.D., Ralph DeBerardinis, M.D., Ph.D., and scientists in CRI’s Genetic and Metabolic Disease Program compared the chemical makeup of skin fibroblasts from healthy subjects to patients with genetic diseases affecting the ETC and related mitochondrial pathways. They discovered ETC impairment suppresses the de novo pathway cells use to build purines from scratch and forces cells to rely on the salvage pathway, where cells re-synthesize purines from partially degraded components.
Researchers also examined metabolism of lung tumors growing in patients and found that tumors with low mitochondrial activity express high levels of the salvage enzyme hypoxanthine phosphoribosyl transferase 1 (HPRT1) and the HPRT1 product inosine monophosphate.
Because ETC dysfunction forced cells to change how they produce purines, it also determined how they respond to inhibitors of purine synthesis. Lung cancer cells with high ETC activity could tolerate HPRT1 inhibition, but cells with low ETC activity became highly dependent on HPRT1. They also became dependent on transporters that allow cells to take up purine bases in the environment to feed salvage reactions.
“The salvage pathway uses less energy than the de novo pathway, possibly explaining why cells with ETC defects prefer to use it. Tumors reprogram their metabolism to sustain growth in the face of environmental challenges,” Dr. Wu said. “Understanding metabolic adaptions to stress within tumors may help us exploit metabolic vulnerabilities and develop better ways to treat cancer.”
“We know that lung tumors growing in patients have variable levels of mitochondrial activity, presumably because the ETC works well in some tumors and poorly in others,” added Dr. DeBerardinis. “We also know that lung tumors respond variably to drugs that block de novo purine synthesis. Our research suggests a reason why these drugs work in some but not all patients and may help us predict which tumors will respond.”
Dr. DeBerardinis extensively studies pediatric inborn errors of metabolism (IEMs), using metabolomics and genomics to identify new disease genes and deepen understanding of metabolism’s role in human health.
“Although cancer and IEMs are clinically very different, many of the same metabolic pathways are altered in both diseases. This study shows how analyzing metabolic differences in IEMs can help us understand the metabolic processes that allow tumors to grow. We think the opposite is also true – pathways that become activated in tumors might help us devise ways to understand and treat IEMs,” Dr. DeBerardinis said.
Dr. Wu is a postdoctoral fellow in CRI’s DeBerardinis lab.
Dr. DeBerardinis is a Howard Hughes Medical Institute (HHMI) Investigator, CRI Professor and Director of CRI’s Genetic and Metabolic Disease Program (GMDP). He is the Robert L. Moody, Sr. Faculty Scholar at CRI and the Joel B. Steinberg M.D. Distinguished Chair in Pediatrics at UT Southwestern.
Research was supported by HHMI and grants from the National Cancer Institute. Patient samples were obtained from CRI’s GMDP, Pascale de Lonlay, M.D., and the Undiagnosed Disease Network.