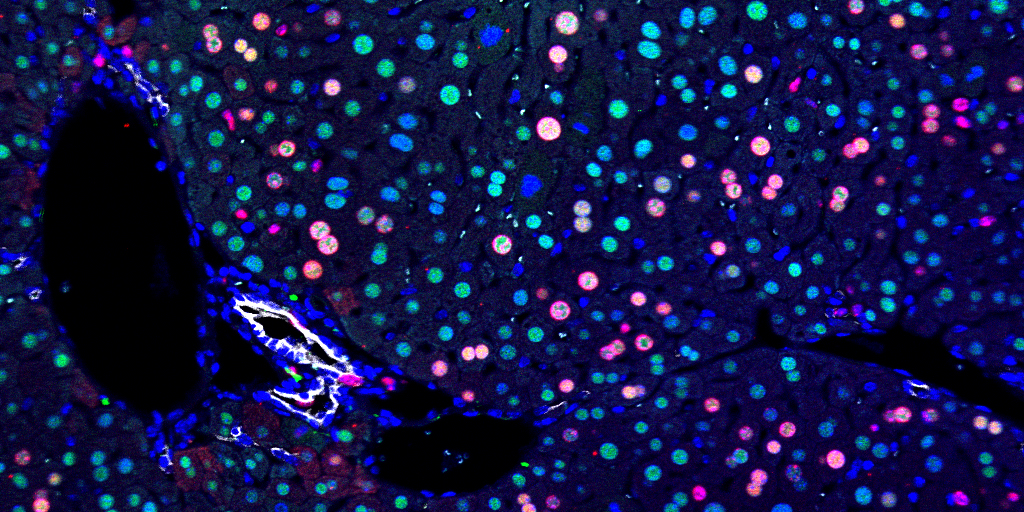
Researchers used fluorescents on a regenerative liver. Blue is DAPI, a nuclear marker. Green is HNF4α, a hepatocyte marker. Red is BrdU, marking proliferating cells. White is CK19, a bile duct marker.
Mishra Lab pinpoints PDK4 enzyme that starves cells with mitochondrial damage

CRI Associate Professor Prashant Mishra, M.D., Ph.D., (left) and Mishra Lab postdoctoral fellow Xun Wang, Ph.D.
(NEWS RELEASE) Liver cells have a vital metabolic inflexibility during regeneration to starve dysfunctional cells and keep damage from spreading, according to new research from Children’s Medical Center Research Institute at UT Southwestern (CRI) published in Science.
CRI Associate Professor Prashant Mishra, M.D., Ph.D., Xun Wang, Ph.D., and colleagues have found that hepatocytes, the cells responsible for most liver function, normally use their mitochondria to process fatty acids, a key energy source during regeneration. When their mitochondria are damaged, hepatocytes turn on PDK4 – a metabolic enzyme that restricts cells from shifting to an alternative energy source – and cells die.
“There are good and bad sides of metabolic flexibility. Although metabolic flexibility has been largely described as beneficial because it gives cells the ability to tolerate shifting environments or alternative nutritional sources, our findings suggest flexibility can also be detrimental by allowing damaged cells to survive,” Dr. Mishra said. “With mitochondrial damage, liver cells actively suppress flexibility – a good thing if it prevents the damage from spreading.”
CRI scientists initially studied the mitochondria of healthy liver cells, both under normal and regenerative conditions. Their analyses showed fatty acids from other parts of the body were transported through the blood to the liver to fuel regeneration. When researchers blocked fatty acid transit, heathy livers were flexible and shifted to other energy sources, including sugars like glucose.
Researchers then examined livers from mice with mutations in their mitochondrial genes. Damaged liver cells were unable to use fatty acids during regeneration and did not shift to other energy sources, preventing livers from regenerating.
To understand why the flexibility was suppressed by mitochondrial mutations, Mishra Lab members examined genes that control a cell’s ability to use alternate energy sources. Results showed increased levels of the PDK4 gene – a negative regulator of a pathway needed to generate energy from glucose. When researchers blocked PDK4, damaged cells in the liver became metabolically flexible and were able to use other energy sources to spread and duplicate.
“The liver has an amazing capacity to regenerate after injury, but it is important that only the healthy cells multiply. We found that metabolic inflexibility can be useful by eliminating unhealthy cells,” Dr. Mishra said.
Previous Mishra Lab research showed that healthy mitochondria are critical in the liver for proper organ function and fatty acid metabolism. This new research illustrates how regenerating livers regulate cell proliferation and how disabling the key regulator PDK4 results in unhealthy livers with fat accumulation and steatosis, also commonly known as fatty liver disease.
“Mitochondrial damage is often observed in common human diseases, including cancer,” Dr. Mishra said. “We hope that by identifying the mechanisms cells use to prevent damage from spreading, we can harness these processes to potentially combat disease and prolong health.”
Dr. Mishra is a member of CRI’s Genetic and Metabolic Disease Program (GMDP), CRI’s Tissue Regeneration Program (TRP), and the Cellular Networks in Cancer Research Program of the Harold C. Simmons Comprehensive Cancer Center at UT Southwestern. He collaborated on this research with GMDP Director Ralph DeBerardinis, M.D., Ph.D., and TRP Director Hao Zhu, M.D.
Dr. Mishra is also an Associate Professor of Pediatrics at UT Southwestern. His research focuses on how to improve understanding and find new insights into mitochondrial diseases in order to develop clinical tools and therapeutic options.
Dr. DeBerardinis is a Professor in CRI, in the Eugene McDermott Center for Human Growth and Development at UTSW, and of Pediatrics, and he co-leads the Cellular Networks in Cancer Research Program of the Simmons Cancer Center. He holds the Joel B. Steinberg, M.D. Distinguished Chair in Pediatrics and is a Sowell Family Scholar in Medical Research. Dr. DeBerardinis is also a Howard Hughes Medical Institute Investigator.
Dr. Zhu is a Professor in CRI, of Internal Medicine and Pediatrics, and he co-leads the Development and Cancer Research Program of the Simmons Cancer Center. He holds the Nancy B. and Jake L. Hamon Distinguished Chair in Therapeutic Oncology Research.
Dr. Wang is an Assistant Instructor in CRI’s Mishra Lab.
Research was supported by grants from the National Institutes of Health, National Cancer Institute, Moody Medical Research Institute, National Science Foundation, Human Frontier Science Program, Pollack Foundation, Simmons Comprehensive Cancer Center Cancer & Obesity Translational Pilot Award, and an Emerging Leader Award from the Mark Foundation for Cancer Research.
About CRI
Children’s Medical Center Research Institute at UT Southwestern (CRI) is a joint venture of UT Southwestern Medical Center and Children’s Medical Center Dallas. CRI’s mission is to perform transformative biomedical research to better understand the biological basis of disease. Located in Dallas, Texas, CRI is home to interdisciplinary groups of scientists and physicians pursuing research at the interface of regenerative medicine, cancer biology and metabolism.
X/Twitter | LinkedIn | Instagram | YouTube | Facebook | Website
About UT Southwestern Medical Center
UT Southwestern, one of the nation’s premier academic medical centers, integrates pioneering biomedical research with exceptional clinical care and education. The institution’s faculty members have received six Nobel Prizes and include 25 members of the National Academy of Sciences, 21 members of the National Academy of Medicine, and 14 Howard Hughes Medical Institute Investigators. The full-time faculty of more than 3,200 is responsible for groundbreaking medical advances and is committed to translating science-driven research quickly to new clinical treatments. UT Southwestern physicians provide care in more than 80 specialties to more than 120,000 hospitalized patients, more than 360,000 emergency room cases, and oversee nearly 5 million outpatient visits a year.