As we age, our muscle stem cells accumulate mitochondrial mutations that undermine the function of the electron transport chain. Mitochondria are the part of the cell that generates energy, and this requires a set of proteins called the electron transport chain, which is required to convert sugar and other nutrients into energy. Damage to the electron transport chain damages muscle fibers. How muscle stem cells handle mitochondrial dysfunction has remained a mystery until now – new research from the Mishra lab at CRI suggests existing muscle tissues act as a sponge and remove damaged cells by absorbing them. The study, published in Nature Aging, also found muscle stem cells are required for muscle regeneration and become depleted with age, offering one possible explanation for why our muscles shrink and weaken with age.
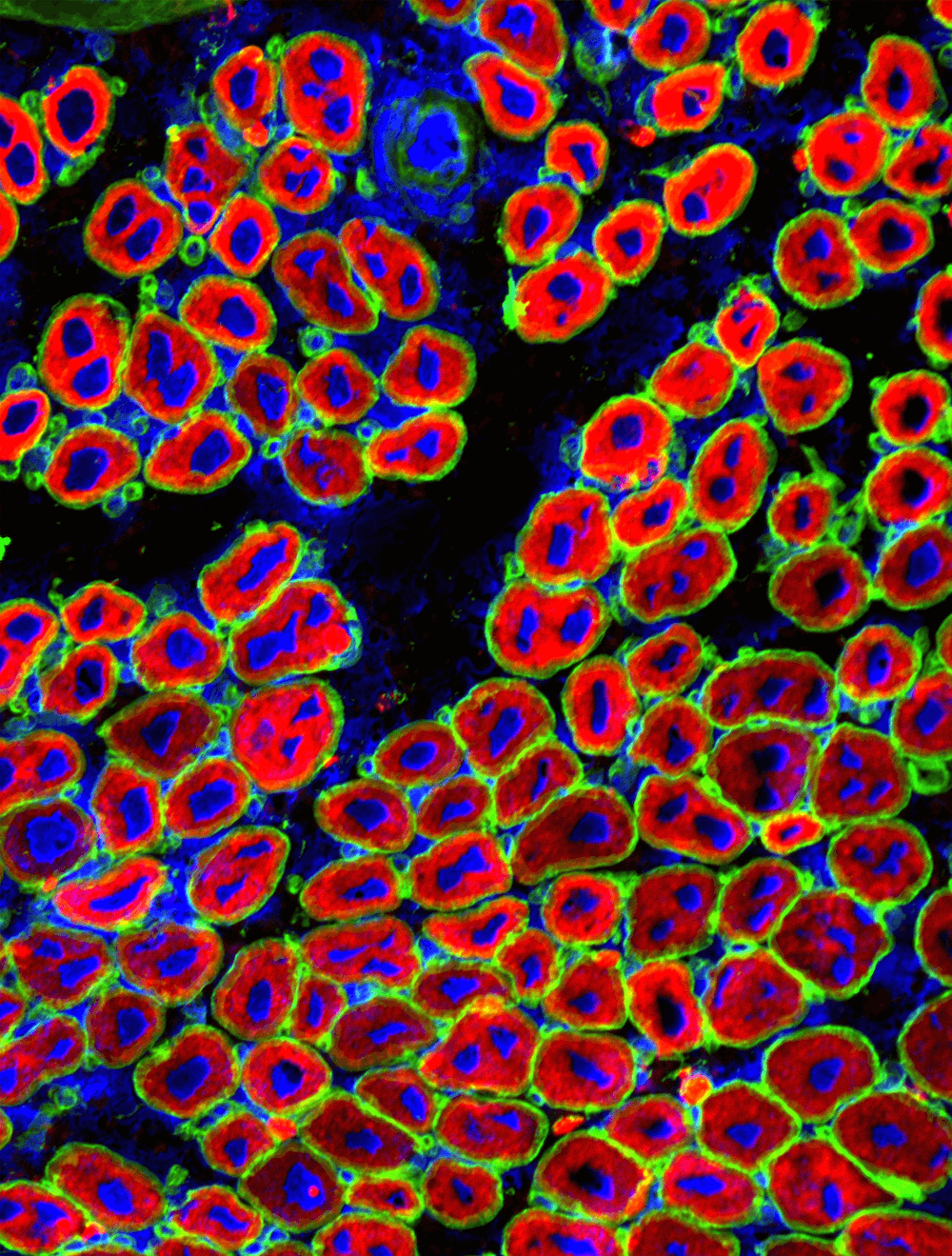
Cross-section of mouse muscle with new/regenerating mouse fibers, stained in red with an antibody against an embryonic myosin heavy chain protein (called Myh3), which were formed from muscle stem cells in response to a chemically induced injury. The green stain labels laminin, which forms the boundary of muscle fibers, and the blue stain (called DAPI) shows the nuclei.
“Unlike mutations that occur in the nucleus, the mitochondria lack the machinery to repair mutations in their genes, so the defective mitochondrial genes have the potential to be replicated and propagated through tissues unless the tissues have a way of eliminating stem cells with defective mitochondria. Until this study, we haven’t understood how different stem cell populations deal with mitochondrial damage,” said Prashant Mishra, M.D., Ph.D., an assistant professor at CRI and lead author of the study.
To investigate how stem cells handle mitochondrial damage, researchers induced mitochondrial dysfunction in the muscle stem cells of mice. They found damaged muscle stem cells fused directly to existing muscle cells, and this process was dependent on the induction of a specific protein, Scinderin. Suppressing or deleting Scinderin stopped the depletion of muscle stem cells during aging, but a consequence of this was that stem cells with dysfunctional mitochondria persisted and continued to generate dysfunctional muscle fibers. Together, these data show that Scinderin plays a vital role in maintaining healthy muscle tissue as we age by clearing damaged cells.
Researchers found mitochondrial DNA mutations accumulate with age when they sequenced the mitochondrial genomes from mice at different ages. Both the accumulation of mutations in the mitochondria and the absorption of damaged muscle stem cells by existing muscle cells could potentially explain why our muscles weaken and shrink with age. Findings from the study also suggest patients with mitochondrial DNA mutations are likely predisposed to having fewer MuSCs since the cells with inherited mitochondrial damage are absorbed at an elevated rate.
“As our bodies age, the number of stem cells in our muscles decrease. Now that we understand this process is related to how these cells deal with mitochondrial damage, we are starting to think of ways to block this process. Ideally, we would like to maintain a large number of healthy stem cells past adulthood – this would allow elderly patients to recover from injuries more quickly,” said Dr. Mishra.