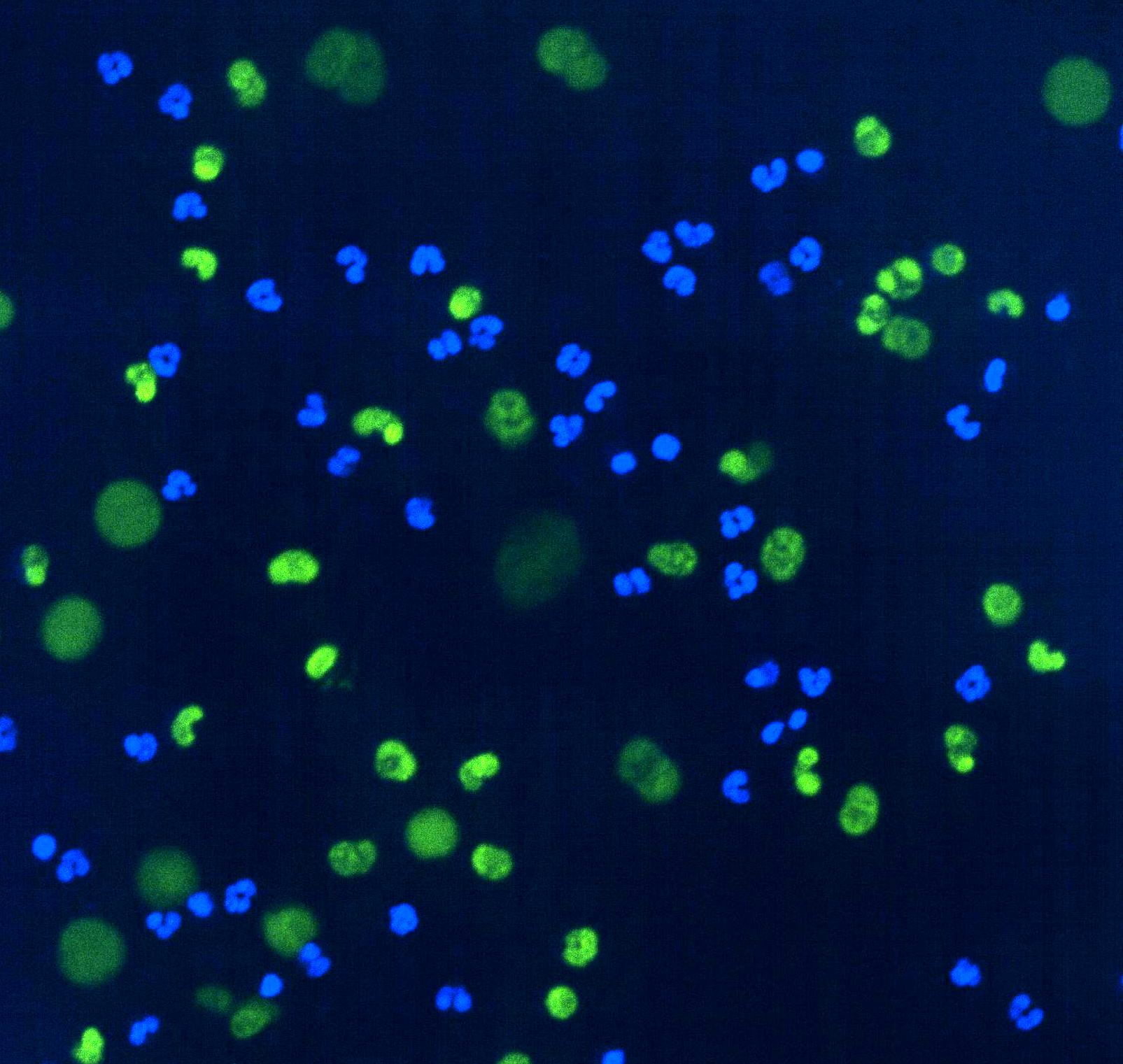
Blue stains indicate the nuclei of live neutrophils. The green ballooning structures show NET formation after GAPDH inhibition.
Metabolic changes in neutrophils, the most abundant type of white blood cell, may contribute to severe COVID-19, according to scientists at Children’s Medical Center Research Institute at UT Southwestern (CRI).
Severe COVID-19 is caused by dysfunction of the immune system in response to SARS-CoV-2 infection. It has been speculated that the metabolism of immune cells could contribute to this dysfunction. However, no one had analyzed the levels of metabolites, the small molecules produced during metabolism, in immune cells from patients. In the study, published in Nature Communications, Yafeng Li, Ph.D., Michalis Agathocleous, Ph.D., and colleagues performed metabolomics analysis of neutrophils isolated from patients with severe or mild COVID-19 and from healthy individuals to understand metabolic changes in severe disease. Researchers identified changes in several metabolic pathways in the cells of severe COVID-19 patients, including pathways not previously implicated in COVID-19 or other inflammatory diseases.
One of these changes was inhibition of a key metabolic enzyme called GAPDH. Scientists in the Agathocleous lab found GAPDH inhibition caused the formation of neutrophil extracellular traps (NETs)— unique structures produced by neutrophils. NETs are essential for containing pathogens but can also damage tissues when responding to many inflammatory diseases, including sepsis, malaria, and stroke, in addition to severe COVID-19.
“Inhibiting GAPDH induced many changes inside neutrophils, including blocking ATP production – the main energy-carrying molecule within cells. Surprisingly, it was not the loss of ATP that caused NET formation; rather, GAPDH controlled NET formation by influencing the cellular pH,” said Dr. Li, an Assistant Instructor at CRI.
According to researchers, the discovery of GAPDH as an innate NET suppressor suggests that preserving GAPDH activity or pH balance in neutrophils could reduce pathology in inflammatory diseases.
“This study provides a first look at the metabolic composition of immune cells in COVID-19. It also suggests that pathological immune cell metabolism contributes to disease severity, provides a resource to understand the metabolism of neutrophils, and opens a new direction to investigate how metabolism affects neutrophil survival and function,” said Dr. Agathocleous, Assistant Professor at CRI, and supervisor of the study.
Dr. Li added, “Our work will stimulate rethinking the role of glycolysis in the hematopoietic and immune systems. This work started with samples from COVID-19 patients, but its implications extend beyond that to understanding the control of NET formation and the metabolic functions of GAPDH.”
Dr. Agathocleous is a Cancer Prevention and Research Institute of Texas (CPRIT) scholar and an American Society of Hematology faculty scholar. This work was supported by CPRIT (RR180007), the American Society of Hematology Faculty Scholar Award, the Welch Foundation (I-2053-20210327), the Moody Foundation, and the National Institutes of Health (R01DK125713 and R01HL161387). Patient samples were collected by the UT Southwestern SARS-CoV-2 Biorepository.