From left: Hao Zhu, M.D., Professor in Children’s Medical Center Research Institute at UT Southwestern (CRI) and of Pediatrics and Internal Medicine at UT Southwestern, and Zhu Lab researchers Roger Liang, B.S., UT Southwestern Perot Family Scholar Medical Scientist Training Program graduate student; Zhijie Li, Ph.D., postdoctoral fellow; and Jason Guo, B.S., UT Southwestern medical student.
Hepatocellular carcinoma arises primarily in zone 3 due to glutathione transferase genes that help mutated cells avoid dying
Scientists have discovered liver cancers arise in specific metabolic zones, where premalignant cells exploit location-specific genes that promote cell survival by chemical detoxification, according to new research from Children’s Medical Center Research Institute at UT Southwestern (CRI) published today in Science.
Liver cells, or hepatocytes, spatially compartmentalize their metabolic functions throughout three zones located in liver lobules, or functional units that comprise the liver. While hepatocytes in different zones have vastly different gene expression, scientists did not previously know where liver cancers most frequently arise.
“The origins of cancer are poorly understood because it is difficult to follow premalignant cells in their native tissue environments. Many cells acquire cancer-causing mutations, but we don’t know why only a small fraction of these premalignant cells develop into cancer,” said Hao Zhu, M.D., Professor in CRI and of Pediatrics and Internal Medicine at UT Southwestern. “It’s important for us to discover which cells and locations give rise to cancers since this can lead us to clues about their preferences and vulnerabilities.”

Andrew Chung, M.D., Ph.D., Massachusetts General Hospital surgery resident and former Zhu Lab researcher.
The study was primarily conducted by Zhu Lab researchers and first authors Jason Guo, B.S., UT Southwestern medical student; Roger Liang, B.S., UT Southwestern Perot Family Scholar Medical Scientist Training Program graduate student; Andrew Chung, M.D., Ph.D., Massachusetts General Hospital surgery resident; and Zhijie Li, Ph.D., Zhu Lab postdoctoral fellow.
Liver damage from a variety of chronic diseases can drive repeated cycles of regeneration, inflammation, fibrosis, and ultimately hepatocellular carcinoma (HCC), the most common form of liver cancer. Using animal models created in the CRI Mouse Genome Engineering Facility directed by Dr. Zhu, CRI researchers genetically introduced the most important liver cancer mutations into hepatocytes in different zones and followed their fates until they turned into cancer.
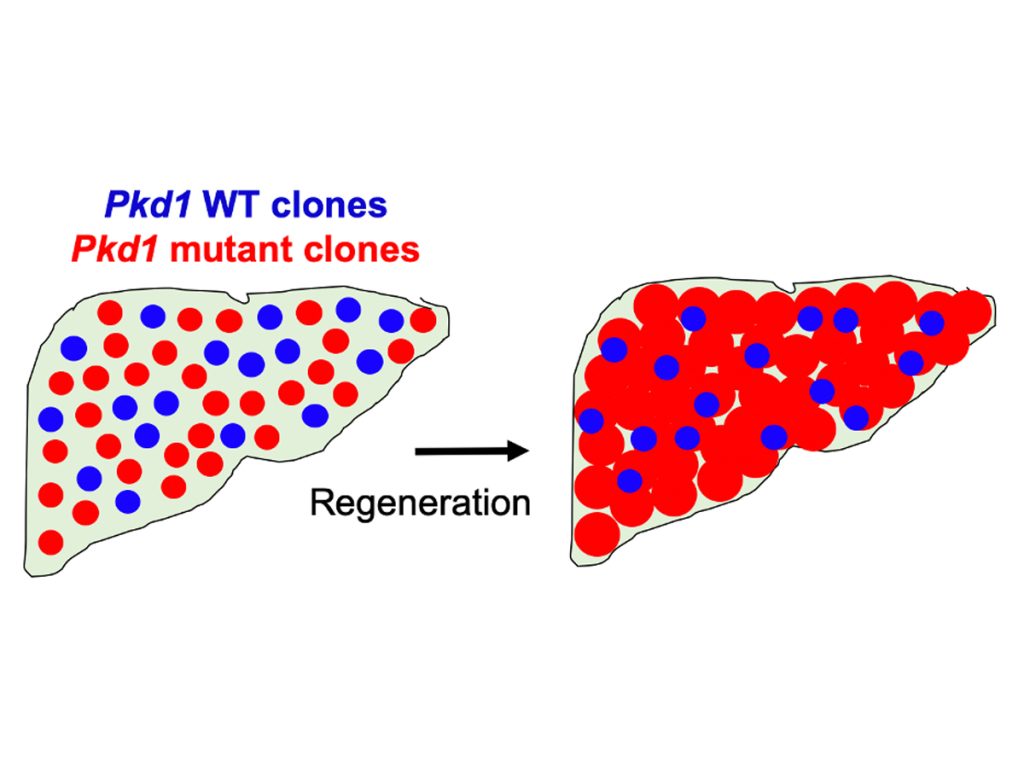
Researchers discovered cells with Ctnnb1 and Arid2 mutations were preserved in zone 1 but disappeared in zone 3. Unexpectedly, Ctnnb1/Arid2-driven cancers were much more likely to arise in zone 3 than in zone 1 in aged mice. This zonal preference was significant because it also applied to other types of liver cancer caused by different mutations.
“The concept of metabolic zonation has been well-studied, but this is one of the first studies that show a ‘tumorigenic zonation,’ which will fundamentally shape our knowledge of liver cancer,” said Mr. Guo, one of the study’s first authors. “If we can identify, for example, the unique zone where a specific mutation may cause cancer, then we can leverage our knowledge of the genes in that zone to find more effective therapies.”
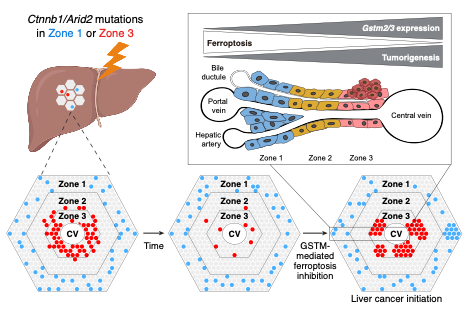
The impact of metabolic zonation in the liver on HCC formation. The lobule is the functional unit of the liver that can be divided into concentric zones. Ctnnb1 and Arid2 double mutant hepatocytes in zone 1 persisted over time while those in zone 3 mostly disappeared. Ultimately, HCCs more frequently arose in zone 3 because GSTM2 and GSTM3 enrichment in this zone led to reduced ferroptosis and increased transformation.
CRI researchers identified two zone 3-enriched genes — glutathione S-transferases Gstm2 and Gstm3 — that helped cells survive during early stages of liver cancer. Glutathione transferase genes help eliminate environmental toxins that cause liver damage. Scientists found high expression of these genes in zone 3 was protecting early-cancer cells from dying from ferroptosis, a specific type of cell death, thereby allowing for tumors to form. By inhibiting Gstm2 and Gstm3 genetically or with drugs, the researchers were able to prevent the formation of liver cancer.
“This discovery alerts us to an important role for ferroptosis in cancer suppression and initiation. We believe ferroptosis could be an important means of preventing widespread cancer development when many cells collect cancer causing mutations,” Dr. Zhu said.
This research builds off prior Zhu Lab research that showcased how mutated cells in the liver could help scientists identify both adaptive and maladaptive genetic changes that occur in disease. Dr. Zhu said his lab plans to continue exploring different cancer-causing genes and how they evolve into cancer in the liver.
Dr. Zhu is the Nancy B. and Jake L. Hamon Distinguished Chair in Therapeutic Oncology Research. He is also Co-leader, Development & Cancer Research Program, Simmons Comprehensive Cancer Center and a Cancer Prevention and Research Institute of Texas Scholar in Cancer Research.
This research was funded by the Pollack Foundation, the National Institutes of Health, the Emerging Leader Award from the Mark Foundation for Cancer Research, an Innovation Award from the Moody Medical Research Institute, an Alpha Omega Alpha Carolyn L. Kuckein Student Research Fellowship, the National Institute of Diabetes and Digestive and Kidney Diseases, CPRIT and a Core Facilities Support Award, the Department of Defense, and the Edward P. Evans Foundation.
About CRI
Children’s Medical Center Research Institute at UT Southwestern (CRI) is a joint venture of UT Southwestern Medical Center and Children’s Medical Center Dallas. CRI’s mission is to perform transformative biomedical research to better understand the biological basis of disease. Located in Dallas, Texas, CRI is home to interdisciplinary groups of scientists and physicians pursuing research at the interface of regenerative medicine, cancer biology, and metabolism — relentless discovery toward the treatments of tomorrow.
X/Twitter | Blue Sky | LinkedIn | Instagram | YouTube | Facebook