 A study by scientists at the Children’s Medical Center Research Institute at UT Southwestern (CRI) is providing insight into the genetic basis of neuropsychiatric disorders. In this research, the first mouse model of a mutation in the arid1b gene was created and then used to show that growth hormone treatments reverse some manifestations of the mutation. The ARID1B gene is one of the most commonly mutated genes in patients with intellectual disability and autism spectrum disorders, but scientists have not yet discerned if and how defects in the ARID1B gene contribute to these clinical manifestations.
A study by scientists at the Children’s Medical Center Research Institute at UT Southwestern (CRI) is providing insight into the genetic basis of neuropsychiatric disorders. In this research, the first mouse model of a mutation in the arid1b gene was created and then used to show that growth hormone treatments reverse some manifestations of the mutation. The ARID1B gene is one of the most commonly mutated genes in patients with intellectual disability and autism spectrum disorders, but scientists have not yet discerned if and how defects in the ARID1B gene contribute to these clinical manifestations.
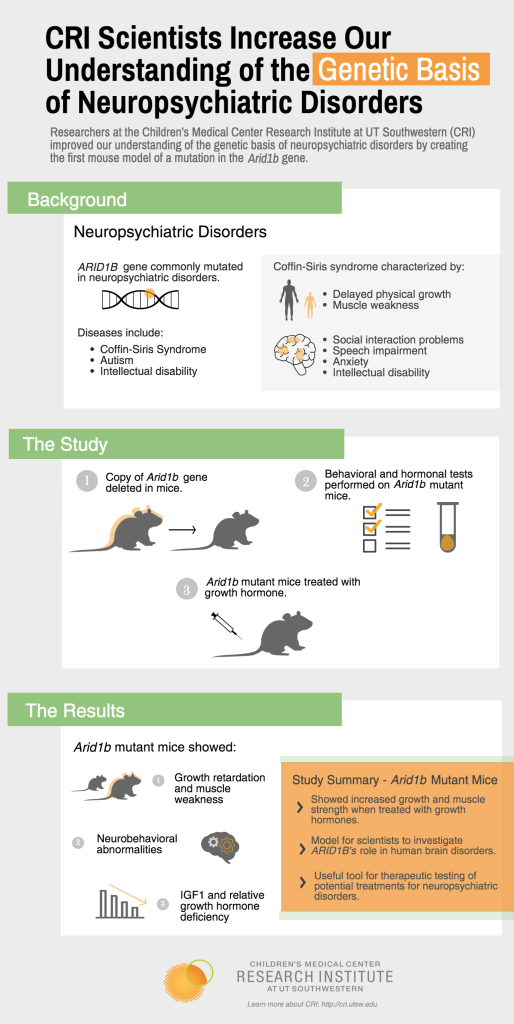
To understand how reduced levels of the protein product of the gene might cause these disorders, a team of researchers led by Dr. Hao Zhu and including graduate student Cemre Celen genetically modified mice to carry a mutation in one of two copies of the ARID1B gene. This mutation replicates the genetics of Coffin-Siris syndrome, a disorder that some patients with defects in the ARID1B gene have that is characterized by speech and social development problems, intellectual disability, and delayed physical growth.
The hope is that by understanding the molecular basis of Coffin-Siris syndrome, scientists will gain a deeper understanding of more common diseases involving intellectual and social impairment.
Scientists found mice with the mutated ARID1B gene exhibited the same type of physical and social changes seen in children with Coffin-Siris syndrome, such as abnormal brain development, muscle weakness, and increased anxiety and fear. The mice also displayed features consistent with autism spectrum disorder, such as social interaction abnormalities, repetitive behaviors, and abnormal “squeaks” or vocalizations. Further testing found these mice had lower-than-expected growth hormone and insulin-like growth factor (IGF1) levels in the blood, potentially explaining the small stature and delayed development seen in human patients. Treating mutant mice with growth hormones restored body size and muscle function, but did not significantly change the behaviors associated with the syndrome.
“These results suggest that growth hormone treatment could be a useful therapy for ARID1B patients. This is an interesting finding because we know some pediatricians already treat Coffin-Siris patients with growth hormones, although they were unaware that this response might be common to many people with ARID1B mutations,” said Dr. Zhu, an Assistant Professor at CRI with joint appointments in Internal Medicine and Pediatrics at UT Southwestern Medical Center and a CPRIT Scholar in Cancer Research.
Dr. Zhu said he believes the study provides the scientific community with an important animal model to further investigate ARID1B’s role in human brain disorders and will be a useful tool for therapeutic testing of potential treatments for autism, intellectual disability, and Coffin-Siris syndrome.
The study was recently published in eLife, an open access journal supported by the Howard Hughes Medical Institute, Max Planck Society, and the Wellcome Trust.
Co-authors included Dr. Jen-Chieh Chuang, former Assistant Instructor at CRI; Dr. Xin Luo, a data scientist at CRI and in Bioinformatics at UTSW; Nadine Nijem, a research technician in the Eugene McDermott Center for Human Growth and Development at UTSW; Dr. Angela Walker, postdoctoral researcher in Neurology and Neurotherapeutics at UTSW; Dr. Fei Chen, research associate at CRI; Shuyuan Zhang and Liem Nguyen, graduate students in the Zhu lab; Andrew Seungjae Chung and Albert Budhipramono, Medical Scientist Training Program students in the Zhu lab; Dr. Ibrahim Nassour, a clinical fellow in surgery at CRI; Dr. Xuxu Sun, Assistant Instructor at CRI and of Pediatrics and Internal Medicine at UTSW; Dr. Shari Birnbaum, Associate Professor of Psychiatry at UTSW; Dr. Craig Powell, Associate Professor of Neurology and Neurotherapeutics, Neuroscience, and Psychiatry at UTSW; Dr. Woo-Ping Ge, Assistant Professor at CRI and of Neuroscience, Neurology and Neurotherapeutics, and Pediatrics at UTSW; and Dr. Maria Chahrour, Assistant Professor in the Eugene McDermott Center for Human Growth and Development and of Neuroscience and Psychiatry at UTSW. Dr. Powell holds the Ed and Sue Rose Distinguished Professorship in Neurology at UT Southwestern.
Other contributors were from the Children’s Hospital of Philadelphia, the University of Pennsylvania, Leiden University Medical Center, Máxima Medical Center, St. George’s University Hospitals, and Queen Mary University of London.
The Cancer Prevention and Research Institute of Texas (CPRIT), the Pollock Foundation, and other donors to the Children’s Medical Center Foundation supported this study.
About CRI
Children’s Medical Center Research Institute at UT Southwestern (CRI) is a joint venture of UT Southwestern Medical Center and Children’s Medical Center, the flagship hospital of Children’s Health. CRI’s mission is to perform transformative biomedical research to better understand the biological basis of disease. Located in Dallas, Texas, CRI is home to interdisciplinary groups of scientists and physicians pursuing research at the interface of regenerative medicine, cancer biology and metabolism. For more information visit: cri.utsw.edu. To support CRI visit: give.childrens.com/about-us/why-help/cri/.