Scientists in the McBrayer lab in Children’s Medical Center Research Institute at UT Southwestern (CRI) have identified a metabolic pathway that can be targeted to stop an aggressive form of brain cancer known as glioma. These findings, published in Cancer Cell, not only offer new insights into the biology of gliomas but also establish the basis of a clinical trial expected to begin next year.
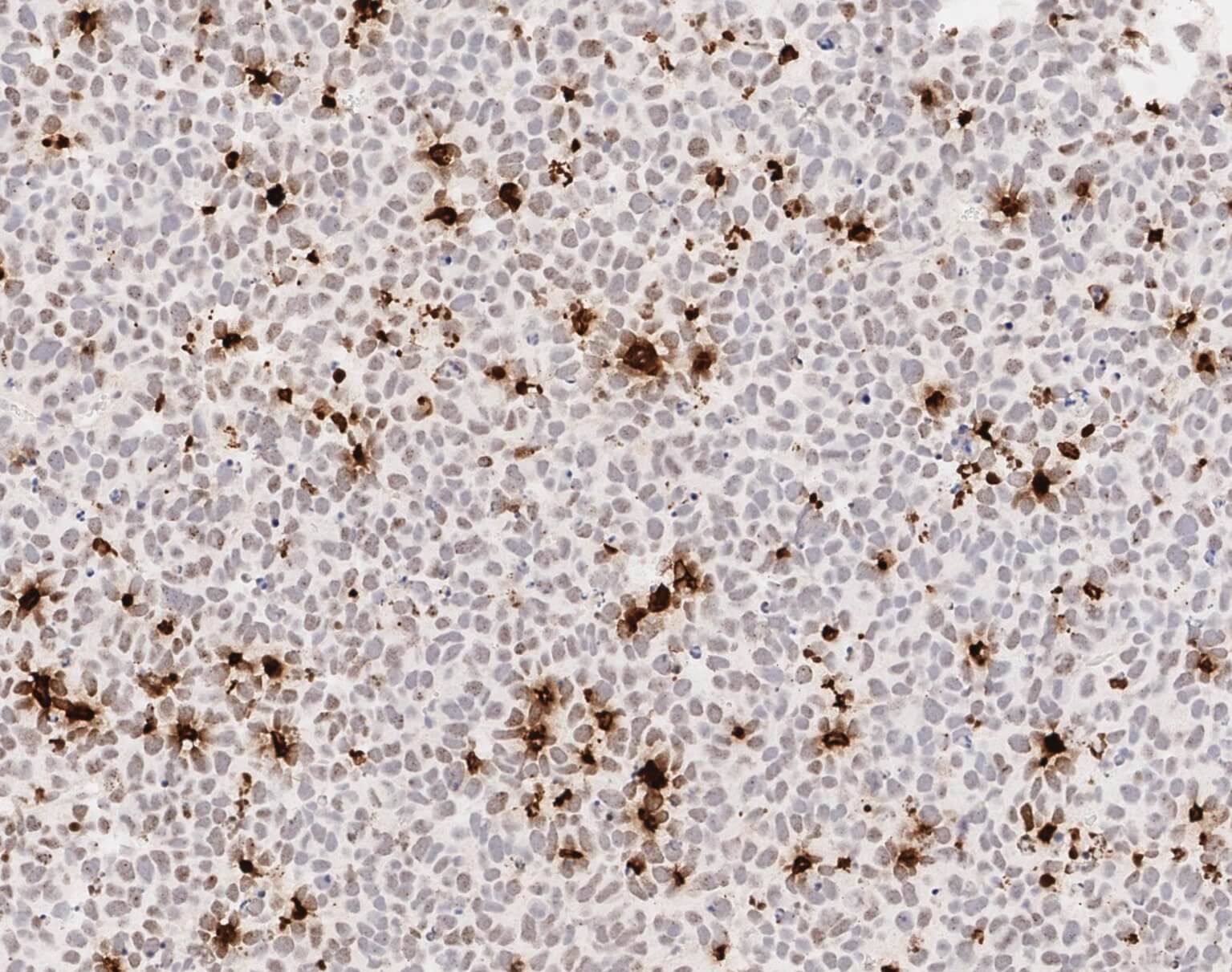
Immunohistochemistry analysis of a DNA damage marker (gamma H2A.X) in an IDH1 mutant glioma xenograft treated with BAY 2402234. Cells stained brown are experiencing DNA damage and express high levels of gamma H2A.X.
Gliomas are among the most lethal and treatment-resistant human cancers. Standard treatment has not changed since 2005, and no new drugs have been approved for glioma therapy in the last decade. A significant portion of gliomas are initiated by mutations that affect IDH genes. These genes encode metabolic enzymes that, when mutated, cause profound metabolic changes in brain cells that cause them to become cancerous. While IDH inhibitors have been used to treat other cancers like leukemia, they are less effective against aggressive gliomas.
To find alternative treatment strategies, scientists in the McBrayer lab screened hundreds of drugs to identify those that preferentially kill glioma cells with IDH mutations. During this process, they discovered that IDH1 mutations increased the dependence of glioma cells on a specific metabolic pathway known as the de novo pyrimidine nucleotide synthesis pathway. Researchers then searched for drugs that could inhibit key enzymes in this pathway in the brain, given that classical inhibitors of this pathway do not readily cross the blood-brain barrier.
Researchers narrowed their search to one particular drug, BAY 2402234, which targets an enzyme called dihydroorotate dehydrogenase (DHODH). Promisingly, this drug accumulated in brain tissue when given to mice, suggesting that it may have utility in treating brain tumors. When researchers tested the drug further, it showed efficacy as a single agent in an array of IDH1 mutant glioma models, including new patient-derived organoids and genetically engineered mouse models of this disease developed by the McBrayer lab earlier this year.
Coincidentally, a team led by Daphne Haas-Kogan, M.D., at the Dana-Farber Cancer Institute independently discovered that this drug also has activity against devastating pediatric brain tumors known as diffuse midline gliomas. Given the potentially broad relevance of these findings for glioma therapy, the studies by Drs. McBrayer and Haas-Kogan will be published back-to-back in the September issue of Cancer Cell.
“These insights hold great promise for the development of precision medicine-based treatment strategies for patients with brain tumors. I’m very grateful for the contributions from our many wonderful collaborators here at CRI, UT Southwestern, and beyond, without whom this multidisciplinary project would not have been feasible. We’re excited about the therapeutic potential of our findings, and look forward to advancing our work to a clinical trial that is planned for next year,” said Samuel McBrayer, Ph.D., an Assistant Professor at CRI and one of the senior authors of the study.
William G. Kaelin, Jr., M.D., professor of medicine at the Dana-Farber Cancer Institute and Harvard Medical School, co-authored the study with Dr. McBrayer. The study was funded by awards from the National Cancer Institute (K22CA237752, R01CA258586, and U19CA264504), the V Foundation for Cancer Research, and Oligo Nation, as well as a gift from the Jonesville Foundation. Dr. McBrayer is a V Foundation Abeloff V Scholar, a recipient of the Sontag Foundation Distinguished Scientist Award, and a Cancer Prevention and Research Institute of Texas Scholar in Cancer Research.