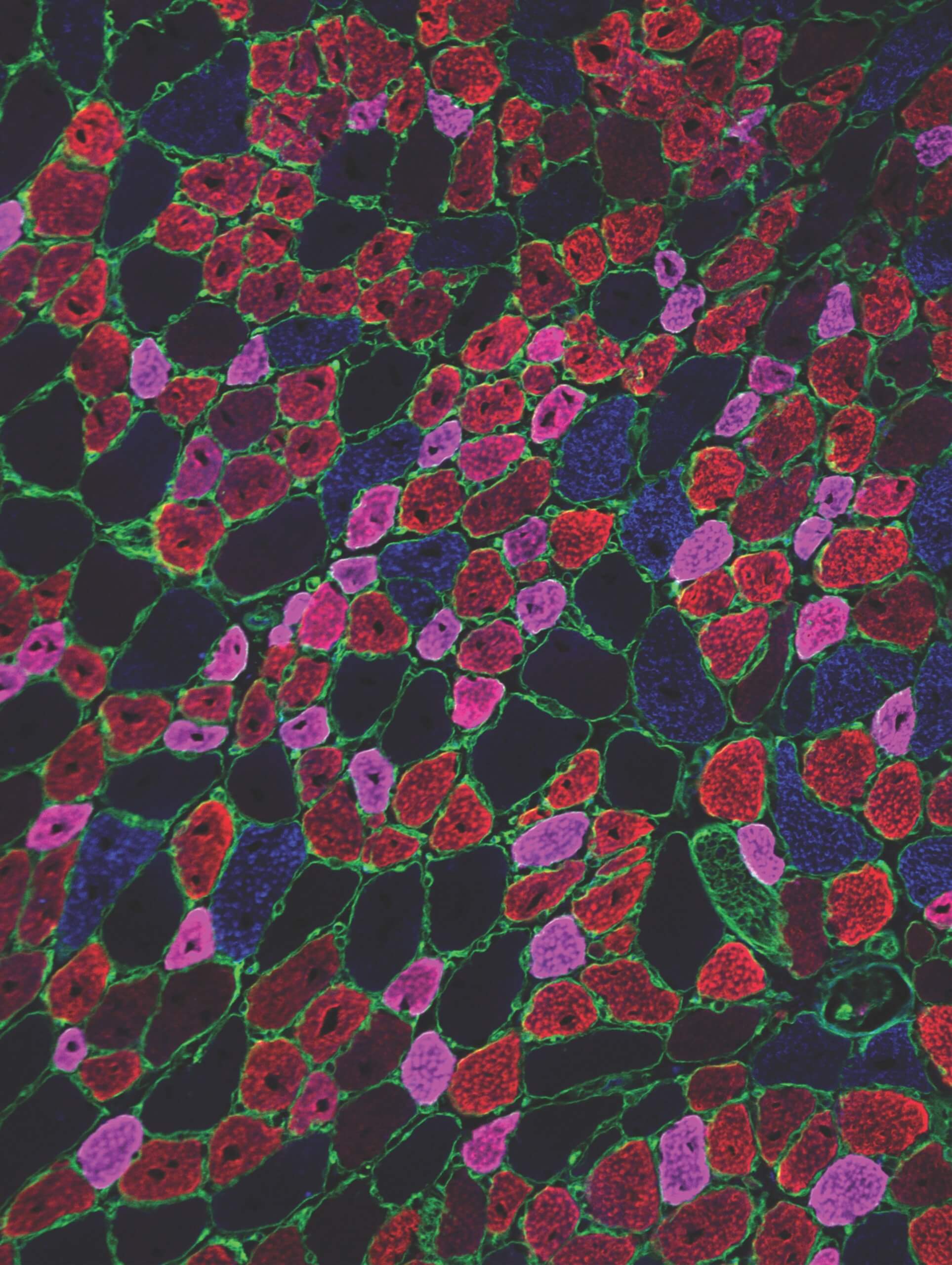
A cross-section of regenerating mouse muscle in which adult myofibers of different types (blue, red, magenta) are identified by immunofluorescent stains.
Recovery time for muscle sprains, strains, and other soft tissue injuries could be shortened thanks to the discovery of a molecular switch that controls how fast muscle tissues regenerate. The study, led by scientists in Children’s Medical Center Research Institute at UT Southwestern (CRI), identified a vital checkpoint that occurs during tissue regeneration. At this checkpoint, muscle fibers are paused at an immature stage for several days before being allowed to continue to develop and grow. Targeting this checkpoint could speed the healing time for patients with traumatic muscle injuries and help patients with diseases like centronuclear myopathy, which causes muscle fibers to permanently pause in an immature state.
“After an injury, muscle stem cells rapidly increase in number and fuse together to make new muscle fibers known as myofibers. Most research in the muscle stem cell field has been focused on the stem cells themselves. How do we make more of them or make them grow faster?” said Prashant Mishra, M.D., Ph.D., an Assistant Professor at CRI and lead author of the study. “We took a different approach and looked at ways to help the newly created myofibers grow and mature faster.”
Focusing on the later stages of muscle regeneration led scientists to several important discoveries. First is the existence of a checkpoint that pauses newly created myofibers at an immature or neonatal state for up to five days before continuing the regeneration process. Second are the two key proteins responsible for regulating this checkpoint—mitochondrial protein Mitofusin 2 (Mfn2) and Hypoxia-induced Factor 1 α (HIF1α). After an injury, Mfn2 is activated by muscle stem cells to help regenerated myofibers grow and mature appropriately. In the absence of Mfn2, HIF1α levels increase substantially, and the newly formed myofibers are frozen in an immature state.
Scientists in the Mishra lab believe this natural checkpoint in the regeneration process is likely a response to hypoxic signaling, which helps synchronize blood vessel formation with tissue regeneration so one does not outpace the other. All newly regenerated tissues need blood vessels to carry oxygen and nutrients to fuel their growth and survival. Interestingly, when researchers inhibited HIF1α and bypassed this checkpoint, they were able to accelerate the regeneration process without any ill effects on the newly formed muscle tissue. Results from the study were published in The Journal of Clinical Investigations.
“Our findings indicate a new way to promote tissue recovery that might synergize with existing therapies targeted at stem cells. In the future, we would like to test this idea with varied types of muscle damage and animal models. If successful, this could lead to an expanded translational approach to shorten a patient’s recovery period from injury,” said Dr. Mishra.